
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 44P
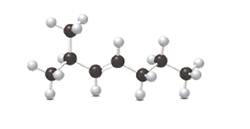
Label the carbon-carbon double bond as cis or trans, and give the IUPAC name for the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In a spontaneous emission process:a) the ground state population decreasesb) the excited state population decreasesc) the non-radiative component is predominantd) the emitted radiation is coherent
For a molecule there are 3 energy levels A, B and C, where B is an intermediate energy level between A and C. The A → C transition occurs at 480 nm and the B → C transition occurs at 885 nm. Indicate the wavelength at which the A → B transition will occur.
For a molecule there are three energy levels: A, B and C. If the transition A → B occurs at 1049 nm and the transition B → C occurs at 885 nm, we can say that the wavelength of the transition A → C will occur at approximately:a) 164 nm b) 1934 nm c) 480 nm d) 967 nm
Chapter 13 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 13.1 - Convert each condensed structure to a complete...Ch. 13.1 - Prob. 13.1PCh. 13.1 - Complete the structure of zingiberene, a component...Ch. 13.2 - Prob. 13.2PPCh. 13.2 - Prob. 13.3PCh. 13.2 - Prob. 13.3PPCh. 13.2 - Prob. 13.4PPCh. 13.3 - Prob. 13.5PPCh. 13.3 - Prob. 13.4PCh. 13.3 - Prob. 13.6PP
Ch. 13.3 - Prob. 13.5PCh. 13.3 - Prob. 13.6PCh. 13.3 - Prob. 13.7PCh. 13.3 - Prob. 13.8PCh. 13.6 - Prob. 13.7PPCh. 13.6 - Prob. 13.9PCh. 13.6 - What products is formed in each of the following...Ch. 13.6 - Prob. 13.9PPCh. 13.6 - What product is formed when l-pentene (CH3CH2CH2CH...Ch. 13.7 - Prob. 13.11PCh. 13.8 - Prob. 13.10PPCh. 13.8 - Prob. 13.12PCh. 13.8 - Prob. 13.13PCh. 13.10 - Prob. 13.11PPCh. 13.10 - Prob. 13.14PCh. 13.11 - Prob. 13.15PCh. 13.12 - Prob. 13.16PCh. 13.13 - Prob. 13.17PCh. 13.13 - Prob. 13.18PCh. 13 - Anethole, the major constituent of anise oil, is...Ch. 13 - Prob. 20PCh. 13 - What is the molecular formula for a hydrocarbon...Ch. 13 - Prob. 22PCh. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - Prob. 26PCh. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each compound. a....Ch. 13 - Give the IUPAC name for each compound. d....Ch. 13 - Give the IUPAC name for each alkene.Ch. 13 - Give the I UP AC name for each alkene.Ch. 13 - Give the IUPAC name for each cyclic compound.Ch. 13 - Give the IUPAC name for each cyclic compound.Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 36PCh. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Label the carbon-carbon double bond as cis or...Ch. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - What alkyd halide is formed when each alkene is...Ch. 13 - Prob. 56PCh. 13 - Prob. 57PCh. 13 - Prob. 58PCh. 13 - What alkene is needed as a starting material to...Ch. 13 - Prob. 60PCh. 13 - Prob. 61PCh. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - Prob. 64PCh. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Prob. 67PCh. 13 - Prob. 68PCh. 13 - Prob. 69PCh. 13 - Prob. 70PCh. 13 - Prob. 71PCh. 13 - Prob. 72PCh. 13 - Prob. 73PCh. 13 - Are o-bromochlorobenzene and m-bromochlorobenzene...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 77PCh. 13 - Prob. 78PCh. 13 - Prob. 79PCh. 13 - Prob. 80PCh. 13 - Prob. 81PCh. 13 - Prob. 82PCh. 13 - Prob. 83PCh. 13 - Prob. 84PCh. 13 - Prob. 85PCh. 13 - Eleostearic acid is an unsaturated fatty acid...Ch. 13 - Prob. 87PCh. 13 - Prob. 88PCh. 13 - Prob. 89PCh. 13 - Prob. 90PCh. 13 - Prob. 91PCh. 13 - Prob. 92PCh. 13 - Prob. 93PCh. 13 - Prob. 94PCh. 13 - Answer the following questions about compound A,...Ch. 13 - Prob. 96PCh. 13 - Answer the following questions about alkene C,...Ch. 13 - Prob. 98PCh. 13 - Prob. 99PCh. 13 - Prob. 100PCh. 13 - Prob. 101CPCh. 13 - Prob. 102CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- : Naming the Alkanes a) Write the IUPAC nomenclature of the compound below b) Draw 4-isopropyl-2,4,5-trimethylheptane, identify the primary, secondary, tertiary, and quaternary carbons. c) Rank pentane, neopentane and isopentane for boiling point. pentane: H3C-CH2-CH2-CH2-CH3 neopentane: CH3 H3C-C-CH3 isopentane: CH3 CH3 H3C-CH2-CH-CH3arrow_forwardAn essential part of the experimental design process is to select appropriate dependent and independent variables. True Falsearrow_forward10.00 g of Compound X with molecular formula C₂Hg are burned in a constant-pressure calorimeter containing 40.00 kg of water at 25 °C. The temperature of the water is observed to rise by 2.604 °C. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound X at 25 °C. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.arrow_forward
- need help not sure what am doing wrong step by step please answer is 971A During the lecture, we calculated the Debye length at physiological salt concentrations and temperature, i.e. at an ionic strength of 150 mM (i.e. 0.150 mol/l) and a temperature of T=310 K. We predicted that electrostatic interactions are effectively screened beyond distances of 8.1 Å in solutions with a physiological salt concentration. What is the Debye length in a sample of distilled water with an ionic strength of 10.0 µM (i.e. 1.00 * 10-5 mol/l)? Assume room temperature, i.e. T= 298 K, and provide your answer as a numerical expression with 3 significant figures in Å (1 Å = 10-10 m).arrow_forwardInfluence of salt concentrations on electrostatic interactions 2 Answer is 2.17A why not sure step by step please What is the Debye length in a concentrated salt solution with an ionic strength of 2.00 mol/l? Assume room temperature, i.e. T= 298 K, and provide your answer as a numerical expression with 3 significant figures in Å (1 Å = 10-10 m).arrow_forwardThe name of the following molecule is: Νarrow_forward
- The table shows the tensile stress-strain values obtained for various hypothetical metals. Based on this, indicate which is the most brittle and which is the most tough (or most resistant). Breaking strength Elastic modulus Material Yield strength Tensile strength Breaking strain A (MPa) 415 (MPa) (MPa) (GPa) 550 0.15 500 310 B 700 850 0.15 720 300 C Non-effluence fracture 650 350arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardMaterials. The following terms are synonyms: tension, effort and stress.arrow_forward
- Please correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardThe table shows the tensile stress-strain values obtained for various hypothetical metals. Based on this, indicate which material will be the most ductile and which the most brittle. Material Yield strength Tensile strength Breaking strain Breaking strength Elastic modulus (MPa) (MPa) (MPa) (GPa) A 310 340 0.23 265 210 B 100 120 0.40 105 150 с 415 550 0.15 500 310 D 700 850 0.14 720 210 E - Non-effluence fracture 650 350arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY