
Concept explainers
What
a.
(a)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:
Concept Introduction:
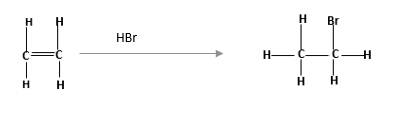
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
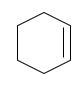
Starting alkene-
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction:
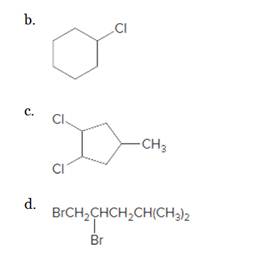
(b)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
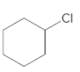
Starting alkene −
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction;
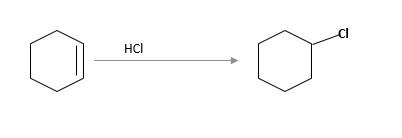
(c)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:
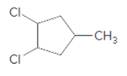
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
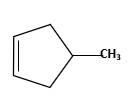 Starting alkene −
Starting alkene −
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction;
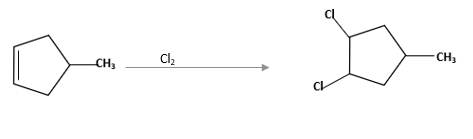
(d)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:

Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
Starting alkene −
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction:
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, and Biological Chemistry - 4th edition
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Organic Chemistry
Fundamentals Of Thermodynamics
Chemistry: Structure and Properties (2nd Edition)
- Label the spectrum with spectroscopyarrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? enantiomers H Br H Br (S) CH3 H3C (S) (R) CH3 H3C H Br A Br H C H Br H3C (R) B (R)CH3 H Br H Br H3C (R) (S) CH3 Br H D identicalarrow_forwardLabel the spectrumarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





