
Concept explainers
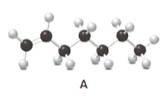
Answer the following questions about compound A, represent in the given ball-and-stick model.
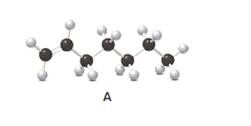
- Convert A to a condensed structure and give its IUPAC name.
- What product is formed when A is treated with H2 in the presence of metal catalyst?
- What product is formed when A is treated with H2O in the presence of H2SO4?
- What
polymer is formed when A ispolymerized ?
(a)
Interpretation:
Structure of the molecule A should be converted to a condensed structure and IUPAC name of the molecule should be given.
 = (
= (
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
In alkene nomenclature, the longest C chain will be considered as the parent/ main C chain. At the end of the alkane suffix -ene is added. The longest chain which includes the carbon-carbon double bond will be considered as the main carbon chain. The numbering of the main C chain is starting from the first substituent by giving the lower number to that substituent and as well as the double bond should get the lower number. All the substituents should be numbered and named. Two or more identical substituents should be indicated by using appropriate prefixes as di-. Names of the substituents should be alphabetized. Each substituent should have a position number which is mentioned in the main C chain.
Answer to Problem 95P
Condensed structure −
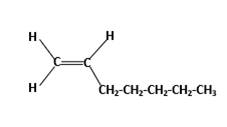
IUPAC name −Heptene
Explanation of Solution
In alkenes, there is a carbon-carbon double bond and different groups are connected to the two carbons. In most of the alkenes; out of four binding positions of carbon-carbon double bond, two of them are bind with two hydrogen atoms and the other two binding positions bind with two different alkyl groups. In this molecule, carbon atoms are represented in black and hydrogen atoms are represented in white color. The structure of a chemical compound is the arrangement of atoms and bonds in the space.
Condensed structure −
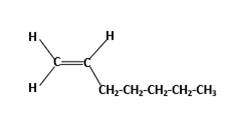
In this molecule, the main carbon chain which contains the carbon-carbon double bond is a seven-membered chain and there are no substituents in this molecule. According to the numbering of the main chain double bond gets the lowest number and it is number 1. Hence, the IUPAC name of the molecule is heptene.
(b)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that contain a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 95P
Explanation of Solution
Unsaturated (
(c)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 95P
Explanation of Solution
Unsaturated (
Refer to the below reaction;
(d)
Interpretation:
The resulting polymer should be identified after polymerization of
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond inside a molecule.
Polymers are high molecular weight macromolecules which formed from covalently bonded monomer molecules.
Answer to Problem 95P
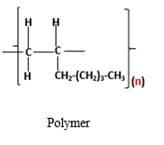
Explanation of Solution
Polymers are high molecular weight macromolecules which formed from covalently bonded monomer molecules. When forming polymers from alkene monomers, the weak bond which is in the carbon-carbon double bond is broken and new strong bond will form to connect the monomer molecules together. Hence; weak bond of the carbon-carbon double bond of

Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, and Biological Chemistry - 4th edition
- 4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward3. 2. 1. On the graph below, plot the volume of rain in milliliters versus its height in centimeters for the 400 mL beaker. Draw a straight line through the points and label it "400 mL beaker." Volume (mL) 400 350 300 250 200 150 750 mL Florence Volume Versus Height of Water 400 mL beaker 100 50 0 0 2 3 4 5 Height (cm) 6 7 8 9 10 Explain why the data points for the beaker lie roughly on a straight line. What kind of relationship is this? How do you know? (see page 276 text) the design of the beaker is a uniform cylinder the volume of liquid increases evenly with its height resulting in a linear relationship. What volume would you predict for 10.0 cm of water? Explain how you arrived at your answer. Use the data table and the graph to assist you in answering the question. 4. Plot the volume of rain in milliliters versus its height in centimeters for the 250 mL Florence flask on the same graph. Draw a best-fit curve through the points and label it "250 mL Florence flask." oke camearrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardIn the video, we looked at the absorbance of a certain substance and how it varies depending on what wavelength of light we are looking at. Below is a similar scan of a different substance. What color BEST describes how this substance will appear? Absorbance (AU) Violet Blue Green Orange 1.2 1.0- 0.8- 0.6- 0.4- 0.2 0.0 450 500 550 600 650 700 Wavelength (nm) violet indigo blue green yellow orange red Red O Cannot tell from this information In the above graph, what causes -450 nm wavelength of light to have a higher absorbance than light with a -550 nm wavelength? Check all that are true. The distance the light travels is different The different data points are for different substances The concentration is different at different times in the experiment Epsilon (molar absortivity) is different at different wavelengthsarrow_forward5. a. Data were collected for Trial 1 to determine the molar mass of a nonvolatile solid solute when dissolved in cyclo- hexane. Complete the table for the analysis (See Report Sheet). Record calculated values with the correct number of significant figures. B. Freezing Point of Cyclohexane plus Calculation Zone Unknown Solute 2. Mass of cyclohexane (g) 10.14 Part C.4 3. Mass of added solute (g) 0.255 C. Calculations 1. k; for cyclohexane (°C⚫ kg/mol) 20.0 2. Freezing point change, AT, (°C) 3.04 Part C.6 3. Mass of cyclohexane in solution (kg) 4. Moles of solute, total (mol) Show calculation. 5. Mass of solute in solution, total (g) 6. Molar mass of solute (g/mol) Show calculation.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


