
Concept explainers
(a)
Interpretation:
Each pair from the following structures should be identified as constitutional isomers, stereoisomers or identical.
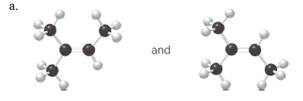
Concept Introduction:
Isomers are the different molecules which have the same molecular formula but differ in molecular arrangement in the space. There are several groups of isomers.
Stereoisomers are the form of isomers which have different three-dimensional orientations in the space.
Constitutional isomers are the form of isomers which have atoms bonded to each other in different ways.
(b)
Interpretation:
Each pair from the following structures should be identified as constitutional isomers, stereo isomers or identical.
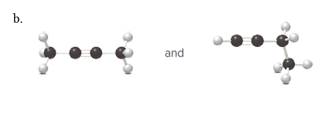
Concept Introduction:
Isomers are the different molecules which have the same molecular formula but differ in molecular arrangement in the space. There are several groups of isomers.
Stereoisomers are the form of isomers which have different three-dimensional orientations in the space.
Constitutional isomers are the form of isomers which have atoms bonded to each other in different ways.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
General, Organic, and Biological Chemistry - 4th edition
- 20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forwardno Ai walkthroughsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





