
Concept explainers
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
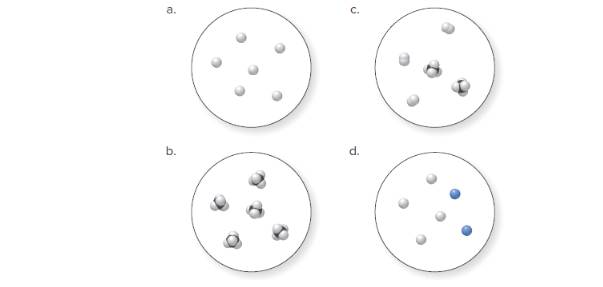
(a)
Interpretation:
Whether the given molecular art is a pure compound, mixture, or a pure element needs to be classified.
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Molecular art 'a' − Pure element.
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now, based on these definitions, molecular art (a) signifies pure elements.
(b)
Interpretation:
Whether the below molecular art is pure compound, mixture, or a pure element needs to be determined.
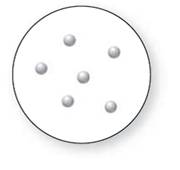
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Molecular art 'b' − Pure compounds
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now based on these definitions, molecular art (b) signifies pure compounds.
(c)
Interpretation:
Whether the below compound is a pure compound, mixture, or a pure element needs to be determined.
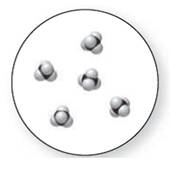
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Molecular art 'c' − Mixture
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now based on these definitions, molecular art (c) signifies mixture.
(c)
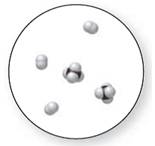
Interpretation:
Whether the below compound is a pure compound, mixture, or a pure element needs to be determined.
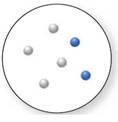
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 19P
Mixture
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now, based on these definitions, molecular art (d) signifies mixture.
Want to see more full solutions like this?
Chapter 1 Solutions
General, Organic, and Biological Chemistry - 4th edition
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Organic Chemistry
Campbell Biology (11th Edition)
- Draw the Lewis structure of C2H4Oarrow_forwarda) 5. Circle all acidic (and anticoplanar to the Leaving group) protons in the following molecules, Solve these elimination reactions, and identify the major and minor products where appropriate: 20 points + NaOCH3 Br (2 productarrow_forwardNonearrow_forward
- Dr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forwardExperiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forward
- Illustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forwardDraw the following molecule: (Z)-1-chloro-1-butenearrow_forwardIdentify the molecule as having a(n) E, Z, cis, or trans configuration. CH3 H₁₂C ○ E ○ z ○ cis transarrow_forward
- Identify the molecule as having a(n) E, Z, cis, or trans configuration. H₂C- CH3 О Е ○ cis ○ transarrow_forwardThe decomposition of dinitrogen pentoxide according to the equation: 50°C 2 N2O5(g) 4 NO2(g) + O2(g) follows first-order kinetics with a rate constant of 0.0065 s-1. If the initial concentration of N2O5 is 0.275 M, determine: the final concentration of N2O5 after 180 seconds. ...arrow_forwardDon't used hand raitingarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





