
Concept explainers
Interpretation:
Boiling point of water and ethyl alcohol must be found at 1 atm from the given figure.
Also, the boiling point of water must be estimated at 0.8 atm pressure.
Concept introduction:
Boiling point of a liquid is the temperature at which vapor pressure of the liquid is equal to the atmospheric pressure.
Answer to Problem 90A
Boiling point of water at 1 atm is 100°C.
Boiling point of ethyl alcohol at 1 atm pressure is 78°C.
Boiling point of water at 0.8 atm is approximately 96°C.
Explanation of Solution
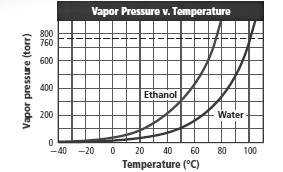
To get the boiling point the corresponding temperature must be found from the graph at which the vapor pressure is equal to the atmospheric pressure (760 torr).
The graph shows that vapor pressure reaches to 760 torr for water and ethyl alcohol at 100°C and 78°C respectively.
So boiling point of water and ethyl alcohol are 100°C and 78°C respectively.
Temperature corresponding to 0.8 atm (608 torr) is 96°C. Thus estimated boiling point of water at 0.8 atm is 96°C.
Boiling point of water and ethyl alcohol are 100°C and 78C respectively.
Estimated boiling point of water at 0.8 atm is 96°C.
Chapter 12 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Chemistry: The Central Science (14th Edition)
Applications and Investigations in Earth Science (9th Edition)
Introductory Chemistry (6th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- First image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forwardwhat is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forward
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





