
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
4th Edition
ISBN: 9781260562620
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 52P
GHB is an addictive, illegal recreational drug that depresses the central nervous system and results in intoxication. Identify the 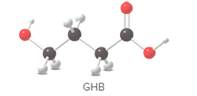
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C.
H₂O(g) + C₁₂O(g) = 2 HOCl(g)
K = 0.0900 at 25°C
с
Calculate the equilibrium concentrations of each gas at 25 °C.
[H₂O]=
[C₁₂O]=
[HOCI]=
M
Σ
M
What units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?
Provide the structure, circle or draw, of the monomeric unit found in the biological polymeric
materials given below.
HO
OH
amylose
OH
OH
행
3
HO
cellulose
OH
OH
OH
Ho
HO
Chapter 11 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
Ch. 11.1 - Prob. 11.1PCh. 11.2 - Fill in all H's and lone pairs in each compound.Ch. 11.3 - Prob. 11.2PPCh. 11.3 - Prob. 11.2PCh. 11.3 - Prob. 11.3PCh. 11.3 - Prob. 11.3PPCh. 11.3 - How many lone pairs are present in lidocaine, the...Ch. 11.4 - Convert each compound to a condensed formula.Ch. 11.4 - Convert each condensed formula to a complete...Ch. 11.4 - Convert each skeletal structure to a complete...
Ch. 11.4 - Prob. 11.5PCh. 11.4 - How many H’s are bonded to each indicated carbon...Ch. 11.4 - Using the skeletal structure, determine the...Ch. 11.5 - Prob. 11.7PCh. 11.5 - Prob. 11.8PCh. 11.5 - For each compound. [1] Identify the functional...Ch. 11.5 - How do a carboxylic acid and an alcohol differ?...Ch. 11.5 - Label each of the following condensed structures...Ch. 11.5 - Prob. 11.11PCh. 11.5 - Prob. 11.12PCh. 11.5 - Identify all of the functional groups in atenolol,...Ch. 11.5 - Prob. 11.13PCh. 11.5 - Prob. 11.10PPCh. 11.5 - Prob. 11.14PCh. 11.6 - Indicate the polar bonds in each compound. Label...Ch. 11.6 - Prob. 11.11PPCh. 11.6 - Prob. 11.16PCh. 11.6 - Predict the water solubility of each compound.Ch. 11.6 - Prob. 11.17PCh. 11.7 - Prob. 11.18PCh. 11.7 - Prob. 11.19PCh. 11.7 - Prob. 11.20PCh. 11 - Prob. 21PCh. 11 - Prob. 22PCh. 11 - Complete each structure by filling in all H’s and...Ch. 11 - Complete the structure of mepivacaine by filling...Ch. 11 - Prob. 25PCh. 11 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - “Ecstasy” is a widely used illegal stimulant....Ch. 11 - Prob. 30PCh. 11 - Explain why each C—C—C bond angle in benzene...Ch. 11 - Prob. 32PCh. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a condensed structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each compound to a skeletal structure.Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each shorthand structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - Convert each skeletal structure to a complete...Ch. 11 - A and B are ball-and-stick models of two compounds...Ch. 11 - Prob. 42PCh. 11 - What is wrong in each of the following shorthand...Ch. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Albuterol (trade names Proventil and Ventolin) is...Ch. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - (a) Identify the functional groups in donepezil,...Ch. 11 - Prob. 51PCh. 11 - GHB is an addictive, illegal recreational drug...Ch. 11 - Prob. 53PCh. 11 - Prob. 54PCh. 11 - Prob. 55PCh. 11 - Prob. 56PCh. 11 - Prob. 57PCh. 11 - (a) Identify the functional groups in venlafaxine,...Ch. 11 - You are given two unlabeled bottles of solids, one...Ch. 11 - State how potassium iodide (KI) and pentane...Ch. 11 - The given beaker contains 100 mL of the organic...Ch. 11 - Prob. 62PCh. 11 - Why do we need to know the shape of a molecule...Ch. 11 - 1,1-Dichloroethylene (CH2=CCl2) is a starting...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Indicate the polar bonds in each molecule. Label...Ch. 11 - Classify each molecule as polar or nonpolar.Ch. 11 - Classify each molecule as polar or nonpolar. a....Ch. 11 - Which molecule is more water soluble? Explain.Ch. 11 - Explain why pantothenic acid, vitamin B5, is water...Ch. 11 - Prob. 71PCh. 11 - Prob. 72PCh. 11 - Explain why regularly taking a large excess of a...Ch. 11 - You can obtain the minimum daily requirement of...Ch. 11 - Prob. 75PCh. 11 - Vitamin B6 is obtained by eating a diet that...Ch. 11 - Prob. 77PCh. 11 - Can an oxygen-containing organic compound, have...Ch. 11 - Prob. 79PCh. 11 - Prob. 80PCh. 11 - Benzocaine is the active ingredient in topical...Ch. 11 - Methyl salicylate is responsible for the...Ch. 11 - Answer the following questions about aldosterone,...Ch. 11 - Answer the following questions about...Ch. 11 - Prob. 85PCh. 11 - Skin moisturizers come in two types, (a) One type...Ch. 11 - THC is the active component in marijuana (Section...Ch. 11 - Cocaine is a widely abused, addicting drug....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forward
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY