
Answer the following questions about levonorgestrel (trade name Plan B). Levonorgestrel interferes with ovulation, the release of an egg from an ovary, so it prevents pregnancy if taken within a few days of unprotected sex.
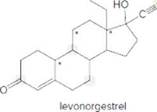
- Identify the
functional groups. - How many C’s does levonorgestrel contain?
- How many H’s are present at each C labeled with an asterisk (*)?
- Give the shape around each atom labeled in gray.
- Label all polar bonds.
- Is levonorgestrel soluble in an organic solvent?
- Is levonorgestrel soluble in water?
(a)
Interpretation:
The functional group in levonorgestral should be identified.
Concept Introduction:
Functional groups are the atoms or group of atoms that gives chemical properties to an organic compound and also chemical reactivity centre. Examples of functional groups are aldehyde, ketone, alcohol, alkene, alkyne etc.
Answer to Problem 84P
The functional groups present in the compound are alcohol, ketone, alkene and alkyne.
Explanation of Solution
Functional groups are the atoms or group of atoms that gives chemical properties to an organic compound and also chemical reactivity centre. Structures of different functional groups are as follows:
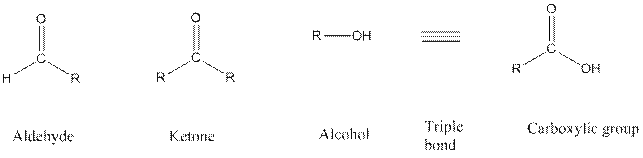
In the above functional group R represents an alkyl group.
In functional group ketone carbonyl carbon is bonded to two alkyl groups, in aldehyde carbonyl carbon is attached to one alkyl group and one hydrogen atom, in alcohol functional group an −OH is bonded to an alkyl group, in alkyne there is a triple bond between two carbon atoms and in alkene there is a double bond between two carbon atoms.
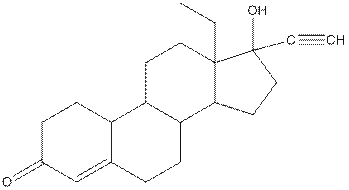
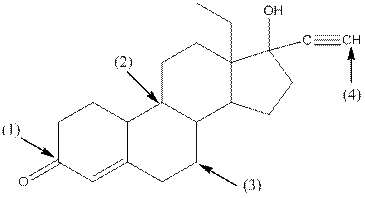
Levonorgestral structure is as follows:
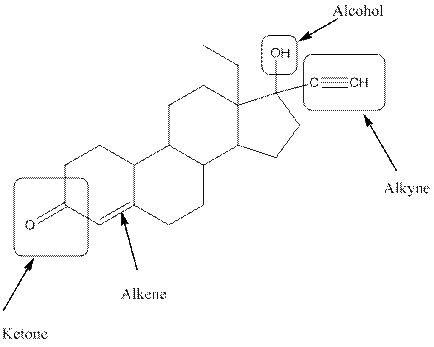
Observe the structure of Levonorgestral. As indicated in the above figure, four types of functional groups present that is, alkene, alkyne, alcohol and ketone. The functional groups present in the compound are alcohol, ketone, alkene and alkyne.
(b)
Interpretation:
The number of carbon atoms in the structure of levonorgestral should be determined.
Concept Introduction:
In skeletal structure the terminals represent methyl
Answer to Problem 84P
Twenty one carbon atoms present in the compound.
Explanation of Solution
The structure of Levonorgestral is as follows:
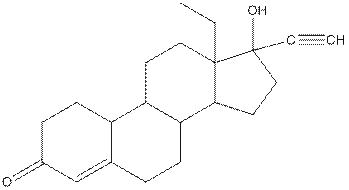
In skeletal structure the terminals represent methyl
Hence, twenty one carbon atoms present in the compound.
(c)
Interpretation:
The number of hydrogen atoms present in the * carbon atoms in levonorgestral should be determined.
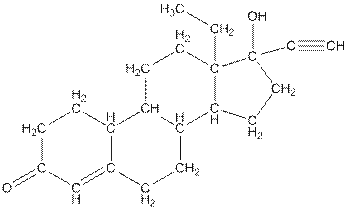
Concept Introduction:
In skeletal structure the terminals represent methyl
Answer to Problem 84P
Number of hydrogen atoms in * carbon atoms are three.
Explanation of Solution
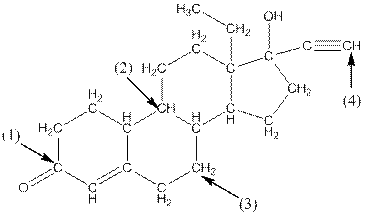
Complete structure of levonorgestral is as follows:
From the complete structure of Levonorgestral, it is clear that number of hydrogen atoms in * carbon atoms are three.
(d)
Interpretation:
The shape around each indicated carbon atom in levonorgestral should be determined.
Concept Introduction:
The following table should be used while determining the shape around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
Answer to Problem 84P
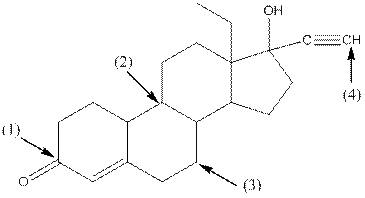
Shape around carbon 1 is trigonal planar, shape around carbon 2 is tetrahedral, shape around carbon 3 is tetrahedral and shape around carbon 4 is linear.
Explanation of Solution
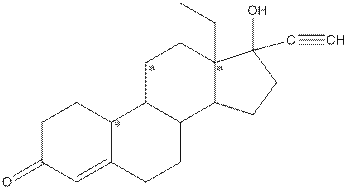
Given compound is as follows:
Complete structure of the compound is as follows:
- Around C1, three atoms (two carbon and one oxygen) present. So, shape around carbon 1 is trigonal planar.
- Around C2, four atoms (three carbon and one hydrogen) present. So, shape around carbon 2 is tetrahedral.
- Around C3, four atoms (two carbon and two hydrogen) present. So, shape around carbon 3 is tetrahedral.
- Around C4, two atoms ( one carbon and one hydrogen) present. So, shape around carbon 4 is linear.
(e)
Interpretation:
All the polar bonds in levonorgestral should be labeled.
Concept Introduction:
The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 84P
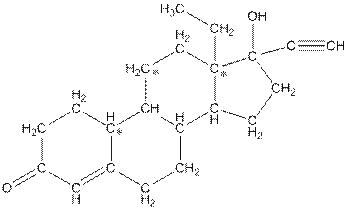
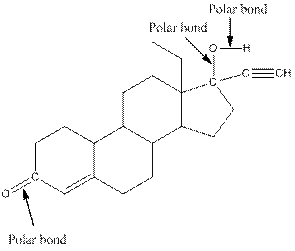
The structure of levonorgestral with labeled all polar bonds is as follows:
Explanation of Solution
In organic compound, most of the polar bonds formed between carbon and heteroatoms like oxygen, nitrogen, sulphur etc.
In the compound, three polar bonds present. One polar bond is between carbon and oxygen where oxygen is more electronegative than carbon. Second polar bond is between carbon and oxygen where oxygen is more electronegative than carbon and third polar bond is between oxygen and hydrogen where oxygen is more electronegative than hydrogen.
The structure of levonorgestral with labeled all polar bonds is as follows:
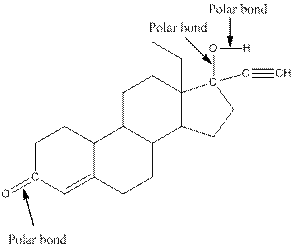
(f)
Interpretation:
Whether levonorgestral is soluble in organic solvent or not should be determined.
Concept Introduction:
A compound is soluble in organic solvent if the compound contains too many hydrocarbon bonds that is if it is non-polar.
Answer to Problem 84P
The compound levonorgestral will soluble in organic solvent.
Explanation of Solution
The complete structure of levonorgestral is as follows:
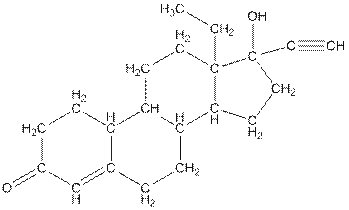
The compound levonorgestral has too many carbon hydrogen bonds. So, the compound will soluble in organic solvent.
(g)
Interpretation:
Whether levonorgestral is soluble in water or not should be determined.
Concept Introduction:
Water is a polar solvent. To dissolve in water the compound must be polar. Polar compound is that compound in which polar bonds are present. The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 84P
The compound levonorgestral is water soluble.
Explanation of Solution
Structure of levonorgestral is as follows:
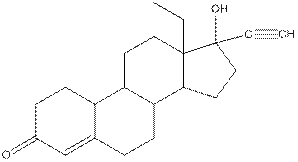
In the compound levonorgestral, two oxygen heteroatoms present. These heteroatoms can form hydrogen bonds with water molecule and hence, the compound is soluble in water.
Want to see more full solutions like this?
Chapter 11 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- The dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forwardSuppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forwardLab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete? Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water? Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?arrow_forward
- What mass of ethylene glycol, HOCH2CH2OH, Mustbe added to 5.50 kg of water to antifreeze that would work for the car radiator to -10.0 degrees celcius? MM (g/mol): 62.07arrow_forwardWhat is the molarity of a 0.393 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 g/molarrow_forwardThe rate constant for the decay of a radioactive element is 2.28 × 10⁻³ day⁻¹. What is the half-life of this element in days?arrow_forward
- Handwritten pleasearrow_forwardChoose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forward
- Choose the best reagents to complete the following reaction. 1. PhNa A 2. H3O+ 1. PhCH2MgBr B 2. H3O+ хё 1. PhMgBr C 2. H3O+ 00 HO Q E D 1. H3O+ 2. PhMgBr PhMgBrarrow_forwardPlease answer all of the questions and provide detailed explanations and include a drawing to show the different signals on the molecule and include which ones should be highlighted.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 1 attempt remaining 1. LiAlH4 2. H3O+ Q OH ☑ Select to Drawarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





