
Concept explainers
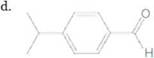
Convert each shorthand structure to a complete structure with all atoms and lone pairs drawn in.
a.
b.
c.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Describe how electronegativity is illustrated on the periodic table including trends between groups and periods and significance of atom size.arrow_forwardDefine the term “transition.” How does this definition apply to the transition metals?arrow_forwardDescribe how the properties of the different types of elements (metals, nonmetals, metalloids) differ.arrow_forward
- Use a textbook or other valid source to research the physical and chemical properties of each element listed in Data Table 1 using the following as a guideline: Ductile (able to be deformed without losing toughness) and malleable (able to be hammered or pressed permanently out of shape without breaking or cracking) or not ductile or malleable Good, semi, or poor conductors of electricity and heat High or low melting and boiling points Occur or do not occur uncombined/freely in nature High, intermediate, or low reactivity Loses or gains electrons during reactions or is not reactivearrow_forwardProvide the Physical and Chemical Properties of Elements of the following elements listedarrow_forwardQuestions 4 and 5arrow_forward
- For a titration of 40.00 mL of 0.0500 M oxalic acid H2C2O4 with 0.1000 M KOH, calculate the pH at each of the following volume of KOH used in the titration: 1) before the titration begin;2) 15 mL; 3) 20 mL; 4) 25 mL; 5) 40 mL; 6) 50 mL. Ka1 = 5.90×10^-2, Ka2 = 6.50×10^-5 for oxalic acid.arrow_forwardPredict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forwardPredict the major organic product(s), if any, of the following reactions. Assume all reagents are in excess unless otherwise indicated.arrow_forward
- How many signals would you expect to find in the 1 H NMR spectrum of each given compound? Part 1 of 2 2 Part 2 of 2 HO 5 ☑ Х IIIIII***** §arrow_forwardA carbonyl compound has a molecular ion with a m/z of 86. The mass spectra of this compound also has a base peak with a m/z of 57. Draw the correct structure of this molecule. Drawingarrow_forwardCan you draw this using Lewis dot structures and full structures in the same way they are so that I can better visualize them and then determine resonance?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





