
Interpretation:
The shape of the labelled atoms in the below structure of enanthotoxin needs to be determined.
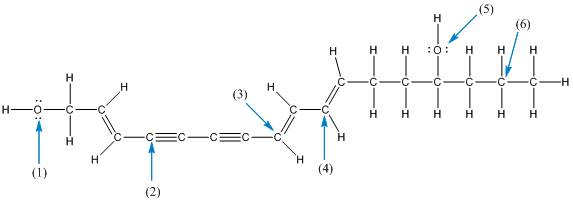
Concept introduction:
Shape of a molecule is determined by including only the bond pair not the lone pairs on the central atom while geometry includes both the bond pairs and lone pairs on the central atom. Valence shell electron pair repulsion theory or VSEPR theory used in chemistry as a model for the prediction of shape of various molecules by knowing the electron pairs on the central atom. There will be repulsion between the electron pairs present on central atom, so to minimize the repulsion they adopt an arrangement with minimum repulsion, thus determining molecule's shape. And by knowing the shape we can easily determine the bond angles.
The following table should be used while determining the shapes:
| Number of groups | Number of lone pairs | Shape | Bond angle | |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- The right-hand side of this reaction shows the product of an aldol condensation. What are the reactants missing from the left-hand side? Draw them below. ? NaOH Δ If there aren't any reactants that would lead to these products under the reaction conditions given, just check the box under the drawing area. Note for advanced students: don't worry if the reactants you propose might also make some other products under these reaction conditions. Just make sure the product above is one of the major products.arrow_forwardPlease help! I need to identify four labeled unknown bottles based off of their colors doing titration using phenlphtalein. I've included my answers, but I wanted to make sure they were correct and if not, what will be correct thank you in advance.arrow_forwardAn organic chemistry Teaching Assistant (TA) suggested in your last discussion section that there is only one major organic product of the following reaction and that this reaction builds a ring. If the TA is right, draw the product in the drawing area below. If the TA is wrong, just check the box below the drawing area. NaOH ?arrow_forward
- A student suggests that the molecule on the right can be made from a single molecule that doesn't have a ring. If the student is correct, draw the starting material below, otherwise, check the box under the drawing area. Click and drag to start drawing a structure. : ☐ + NaOH टेarrow_forwardRate = k [I]1.7303[S2O82-]0.8502, Based on your rate, write down a mechanism consistent with your results and indicate which step is the rate determining step.arrow_forward36. Give the major product(s) of each of the following reactions. Aqueous work-up steps (when necessary) have been omitted. a. CH3CH=CHCH3 b. CH3CH2CH2CCH3 H,PO₂, H₂O, A (Hint: See Section 2-2.) 1. LIAIH. (CH,CH,),O 2. H', H₂O H NaBH, CH,CH₂OH d. Br LIAIH. (CH,CH,)₂O f. CH3 NaBH, CH,CH,OH (CH3)2CH H NaBH, CH,CH₂OH Harrow_forward
- Predict the major products of this reaction: + H excess NaOH Δ ? Note that the second reactant is used in excess, that is, there is much more of the second reactant than the first. If there won't be any products, just check the box under the drawing area instead.arrow_forwardAn organic chemistry Teaching Assistant (TA) suggested in your last discussion section that there is only one major organic product of the following reaction and that this reaction builds a ring. If the TA is right, draw the product in the drawing area below. If the TA is wrong, just check the box below the drawing area. 1. NaOMe CH3O N. OCH3 ? 2. H3O+arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. + More... ☐ ☐ : ☐ + G 1. NaOMe Click and drag to start drawing a structure. 2. H +arrow_forward
- 6. Ammonia reacts with nitrogen monoxide and oxygen to form nitrogen and water vapor. If the rate of consumption of NO is 4.5 mollitermin) (a) Find the rate of reaction (b) Find the rate of formations of N; and HO (c) Find the rate of consumption of NH, and O 4NH: 4NO 0:4: +60arrow_forward34. Give the expected major product of each of the following reactions. Conc. HI a. CH3CH2CH2OH b. (CH3)2CHCH2CH2OH Conc. HBr H Conc. HI C. OH Conc.HCI d. (CH3CH2)3COHarrow_forward42. Which of the following halogenated compounds can be used successfully to prepare a Grignard reagent for alcohol synthesis by subsequent reaction with an aldehyde or ketone? Which ones cannot and why? H3C CH3 a. Br H OH b. Cl C. I H H d. Cl e. H OCH3 Br Harrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





