
Organic And Biological Chemistry
7th Edition
ISBN: 9781305081079
Author: STOKER, H. Stephen (howard Stephen)
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.71EP
Assign an IUPAC name to each of the compounds in Problem 12-63.
a. 
b. 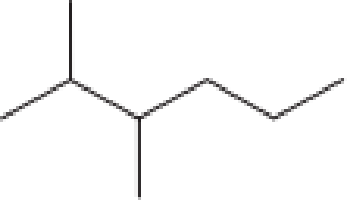
c. 
d. 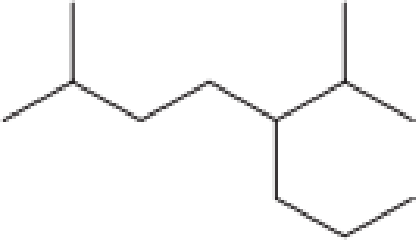
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in
your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on
the LC-MS printout. How much different are they?
2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit,
explain what each of these is and why they are present.
3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by
calculating the accurate monoisotopic mass.
4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum
of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source.
5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one
point of extra credit, see if you can identify this molecule as well and confirm by…
Please draw, not just describe!
can you draw each step on a piece of a paper please this is very confusing to me
Chapter 1 Solutions
Organic And Biological Chemistry
Ch. 1.1 - Prob. 1QQCh. 1.1 - Prob. 2QQCh. 1.2 - Which of the following statements about the...Ch. 1.2 - Prob. 2QQCh. 1.3 - Prob. 1QQCh. 1.3 - Prob. 2QQCh. 1.4 - Prob. 1QQCh. 1.4 - Prob. 2QQCh. 1.4 - Prob. 3QQCh. 1.5 - Prob. 1QQ
Ch. 1.5 - Prob. 2QQCh. 1.5 - Prob. 3QQCh. 1.6 - Prob. 1QQCh. 1.6 - Prob. 2QQCh. 1.6 - Prob. 3QQCh. 1.6 - Prob. 4QQCh. 1.7 - Prob. 1QQCh. 1.7 - Prob. 2QQCh. 1.8 - Prob. 1QQCh. 1.8 - Prob. 2QQCh. 1.8 - Prob. 3QQCh. 1.8 - Prob. 4QQCh. 1.8 - Prob. 5QQCh. 1.8 - Prob. 6QQCh. 1.8 - Prob. 7QQCh. 1.9 - Prob. 1QQCh. 1.9 - Prob. 2QQCh. 1.10 - Prob. 1QQCh. 1.10 - Prob. 2QQCh. 1.11 - Prob. 1QQCh. 1.11 - Prob. 2QQCh. 1.11 - Prob. 3QQCh. 1.12 - Prob. 1QQCh. 1.12 - Prob. 2QQCh. 1.12 - Prob. 3QQCh. 1.13 - Prob. 1QQCh. 1.13 - Prob. 2QQCh. 1.13 - Prob. 3QQCh. 1.14 - Prob. 1QQCh. 1.14 - Prob. 2QQCh. 1.14 - Prob. 3QQCh. 1.15 - Prob. 1QQCh. 1.15 - Prob. 2QQCh. 1.16 - Prob. 1QQCh. 1.16 - Prob. 2QQCh. 1.16 - Prob. 3QQCh. 1.17 - Prob. 1QQCh. 1.17 - Prob. 2QQCh. 1.17 - Prob. 3QQCh. 1.17 - Prob. 4QQCh. 1.18 - Prob. 1QQCh. 1.18 - Prob. 2QQCh. 1.18 - Prob. 3QQCh. 1.18 - Prob. 4QQCh. 1 - Prob. 1.1EPCh. 1 - Prob. 1.2EPCh. 1 - Prob. 1.3EPCh. 1 - Prob. 1.4EPCh. 1 - Indicate whether each of the following situations...Ch. 1 - Indicate whether each of the following situations...Ch. 1 - Prob. 1.7EPCh. 1 - Prob. 1.8EPCh. 1 - What is the difference between a saturated...Ch. 1 - What structural feature is present in an...Ch. 1 - Prob. 1.11EPCh. 1 - Prob. 1.12EPCh. 1 - Prob. 1.13EPCh. 1 - Prob. 1.14EPCh. 1 - Prob. 1.15EPCh. 1 - Prob. 1.16EPCh. 1 - Convert the expanded structural formulas in...Ch. 1 - Prob. 1.18EPCh. 1 - Prob. 1.19EPCh. 1 - Prob. 1.20EPCh. 1 - Prob. 1.21EPCh. 1 - Prob. 1.22EPCh. 1 - Prob. 1.23EPCh. 1 - Prob. 1.24EPCh. 1 - Prob. 1.25EPCh. 1 - Prob. 1.26EPCh. 1 - Indicate whether each of the following would be...Ch. 1 - Indicate whether each of the following would be...Ch. 1 - Prob. 1.29EPCh. 1 - Explain why two different straight-chain alkanes...Ch. 1 - With the help of Table 12-1, indicate how many...Ch. 1 - Prob. 1.32EPCh. 1 - How many of the numerous eight-carbon alkane...Ch. 1 - How many of the numerous seven-carbon alkane...Ch. 1 - For each of the following pairs of structures,...Ch. 1 - For each of the following pairs of structures,...Ch. 1 - Convert each of the following linear condensed...Ch. 1 - Prob. 1.38EPCh. 1 - Prob. 1.39EPCh. 1 - Prob. 1.40EPCh. 1 - Prob. 1.41EPCh. 1 - Prob. 1.42EPCh. 1 - Prob. 1.43EPCh. 1 - Prob. 1.44EPCh. 1 - Prob. 1.45EPCh. 1 - Prob. 1.46EPCh. 1 - Prob. 1.47EPCh. 1 - Prob. 1.48EPCh. 1 - Prob. 1.49EPCh. 1 - Prob. 1.50EPCh. 1 - Prob. 1.51EPCh. 1 - Prob. 1.52EPCh. 1 - Draw a condensed structural formula for each of...Ch. 1 - Draw a condensed structural formula for each of...Ch. 1 - Prob. 1.55EPCh. 1 - For each of the alkanes in Problem 12-54,...Ch. 1 - Explain why the name given for each of the...Ch. 1 - Prob. 1.58EPCh. 1 - Indicate whether or not the two alkanes in each of...Ch. 1 - Prob. 1.60EPCh. 1 - How many of the 18 C8 alkane constitutional...Ch. 1 - How many of the nine C7 alkane constitutional...Ch. 1 - Prob. 1.63EPCh. 1 - Prob. 1.64EPCh. 1 - Prob. 1.65EPCh. 1 - Prob. 1.66EPCh. 1 - Do the line-angle structural formulas in each of...Ch. 1 - Do the line-angle structural formulas in each of...Ch. 1 - Convert each of the condensed structural formulas...Ch. 1 - Convert each of the condensed structural formulas...Ch. 1 - Assign an IUPAC name to each of the compounds in...Ch. 1 - Prob. 1.72EPCh. 1 - Prob. 1.73EPCh. 1 - Prob. 1.74EPCh. 1 - For each of the alkane structures in Problem...Ch. 1 - For each of the alkane structures in Problem...Ch. 1 - Prob. 1.77EPCh. 1 - Prob. 1.78EPCh. 1 - Prob. 1.79EPCh. 1 - Prob. 1.80EPCh. 1 - Prob. 1.81EPCh. 1 - Prob. 1.82EPCh. 1 - Draw condensed structural formulas for the...Ch. 1 - Draw condensed structural formulas for the...Ch. 1 - To which carbon atoms in a hexane molecule can...Ch. 1 - Prob. 1.86EPCh. 1 - Prob. 1.87EPCh. 1 - Prob. 1.88EPCh. 1 - Give an acceptable alternate name for each of the...Ch. 1 - Prob. 1.90EPCh. 1 - Prob. 1.91EPCh. 1 - Prob. 1.92EPCh. 1 - Prob. 1.93EPCh. 1 - Prob. 1.94EPCh. 1 - What is the molecular formula for each of the...Ch. 1 - Prob. 1.96EPCh. 1 - Prob. 1.97EPCh. 1 - Prob. 1.98EPCh. 1 - Prob. 1.99EPCh. 1 - How many secondary carbon atoms are present in...Ch. 1 - Assign an IUPAC name to each of the following...Ch. 1 - Assign an IUPAC name to each of the following...Ch. 1 - Prob. 1.103EPCh. 1 - Prob. 1.104EPCh. 1 - Prob. 1.105EPCh. 1 - Prob. 1.106EPCh. 1 - What is the molecular formula for each of the...Ch. 1 - Prob. 1.108EPCh. 1 - Prob. 1.109EPCh. 1 - Prob. 1.110EPCh. 1 - Prob. 1.111EPCh. 1 - Prob. 1.112EPCh. 1 - Determine whether cistrans isomerism is possible...Ch. 1 - Prob. 1.114EPCh. 1 - Prob. 1.115EPCh. 1 - Prob. 1.116EPCh. 1 - Prob. 1.117EPCh. 1 - Indicate whether the members of each of the...Ch. 1 - Prob. 1.119EPCh. 1 - Prob. 1.120EPCh. 1 - Prob. 1.121EPCh. 1 - Prob. 1.122EPCh. 1 - Prob. 1.123EPCh. 1 - Which member in each of the following pairs of...Ch. 1 - Prob. 1.125EPCh. 1 - Prob. 1.126EPCh. 1 - Answer the following questions about the...Ch. 1 - Prob. 1.128EPCh. 1 - Prob. 1.129EPCh. 1 - Prob. 1.130EPCh. 1 - Write molecular formulas for all the possible...Ch. 1 - Write molecular formulas for all the possible...Ch. 1 - Prob. 1.133EPCh. 1 - Prob. 1.134EPCh. 1 - Prob. 1.135EPCh. 1 - Assign an IUPAC name to each of the following...Ch. 1 - Prob. 1.137EPCh. 1 - Prob. 1.138EPCh. 1 - Prob. 1.139EPCh. 1 - Prob. 1.140EPCh. 1 - Prob. 1.141EPCh. 1 - Prob. 1.142EPCh. 1 - Prob. 1.143EPCh. 1 - Prob. 1.144EPCh. 1 - Prob. 1.145EPCh. 1 - Prob. 1.146EPCh. 1 - Give the IUPAC names for the eight isomeric...Ch. 1 - Prob. 1.148EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forward
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY