
(a)
Interpretation:
The major products of the following reactions should be determined.
Concept Introduction:
Halogen addition to an alkene:
The addition reaction of halides to
Mainly
In general, the reaction can be shown as below.
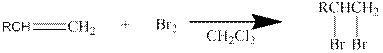
When the bromine approaches the alkene, one bromine accepts the electrons from alkene and give them to the other bromine and a cyclic bromonium ion intermediate is formed.
The formed unstable intermediate reacts with
(b)
Interpretation:
The major products of the following reactions should be determined.
Concept Introduction:
Halogen addition to an alkene:
The addition reaction of halides to alkenes leads to the formation of vicinal dihalide as the product. The two halogens will be on the adjacent carbons.
Mainly
In general, the reaction can be shown as below.
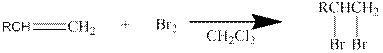
When the bromine approaches the alkene, one bromine accepts the electrons from alkene and give them to the other bromine and a cyclic bromonium ion intermediate is formed.
The formed unstable intermediate reacts with
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
