
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 72P
The second-order rate constant (in units of M–1s–1) for acid-catalyzed hydration at 25 °C is given (or each of the following
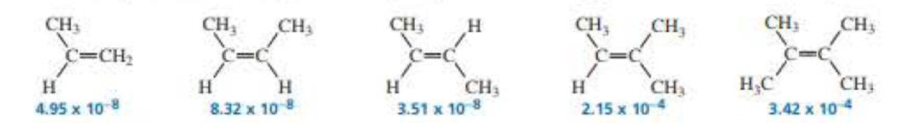
- a. Calculate the relative rotes of hydration of the alkenes. (Hint: Divide each rate constant by the smallest rate constant of the series: 3.51 × 10–8.)
- b. Why does (Z)-2-butene react faster than (E)-2-butene?
- c. Why does 2-methyl-2-butene react faster than (Z)-2-butene?
- d. Why does 2.3-dimethyl-2-butene react faster than 2-melhyl-2·butene?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the functions that salt bridges have in batteries.
In the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.
Write the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are avilable for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.4 - Prob. 6PCh. 6.4 - Prob. 7PCh. 6.4 - What alkene should be used to synthesize each of...Ch. 6.5 - The pKa of a protonated alcohol is about 2.5, and...Ch. 6.5 - Prob. 10P
Ch. 6.5 - Prob. 11PCh. 6.6 - a. What is the major product or each or the...Ch. 6.6 - Prob. 14PCh. 6.6 - Prob. 15PCh. 6.7 - What is the major product obtained from the...Ch. 6.8 - Which is more highly regionselective: reaction of...Ch. 6.8 - Prob. 19PCh. 6.9 - What will be the product of the preceding reaction...Ch. 6.9 - Prob. 21PCh. 6.9 - Prob. 22PCh. 6.9 - Prob. 23PCh. 6.9 - What is the product of the addition of 1Cl to...Ch. 6.9 - What will be the major product obtained from the...Ch. 6.9 - Propose a mechanism for the following reaction:Ch. 6.10 - Draw structures for the following: a. 24...Ch. 6.10 - What alkene would you treat with a peroxyacid in...Ch. 6.11 - What products are formed when the following...Ch. 6.11 - Prob. 31PCh. 6.11 - Prob. 32PCh. 6.11 - The following product was obtained from the...Ch. 6.12 - What characteristics must the reactant of a...Ch. 6.13 - Prob. 36PCh. 6.13 - What stereoisomers are obtained from each of the...Ch. 6.13 - Prob. 41PCh. 6.13 - Prob. 42PCh. 6.13 - Prob. 43PCh. 6.13 - Prob. 45PCh. 6.13 - Prob. 46PCh. 6.13 - Prob. 47PCh. 6.13 - Prob. 48PCh. 6.13 - Prob. 49PCh. 6.13 - Prob. 50PCh. 6.14 - Prob. 51PCh. 6.16 - Prob. 53PCh. 6.16 - Explain why 3-methykyclohexene should not be used...Ch. 6 - Prob. 55PCh. 6 - Prob. 56PCh. 6 - Prob. 57PCh. 6 - What is the major product of the reaction of...Ch. 6 - Give two names for each of the following:Ch. 6 - Prob. 60PCh. 6 - What are the products of the following reactions?...Ch. 6 - When 3-methyl-1-butene reacts with HBr, two alkyl...Ch. 6 - Draw curved arrows to show the flow of electrons...Ch. 6 - What reagents are needed to carry out the...Ch. 6 - Prob. 65PCh. 6 - Prob. 66PCh. 6 - Prob. 67PCh. 6 - What is more stable? a. CH3C+HCH3orCH3C+HCH2ClCh. 6 - Prob. 69PCh. 6 - a. Draw the product or products that will be...Ch. 6 - Prob. 71PCh. 6 - The second-order rate constant (in units of M1s1)...Ch. 6 - Which compound has the greater dipole moment?Ch. 6 - Prob. 74PCh. 6 - Prob. 75PCh. 6 - Prob. 76PCh. 6 - Prob. 77PCh. 6 - Prob. 78PCh. 6 - Prob. 79PCh. 6 - Prob. 80PCh. 6 - Prob. 81PCh. 6 - Prob. 82PCh. 6 - Prob. 83PCh. 6 - Prob. 84PCh. 6 - Prob. 85PCh. 6 - Prob. 86PCh. 6 - Draw the products of the following reactions. If...Ch. 6 - Prob. 88PCh. 6 - Prob. 89PCh. 6 - Prob. 90PCh. 6 - Two chemists at Dupont found that lCH2Znl is...Ch. 6 - Prob. 92PCh. 6 - Prob. 93PCh. 6 - What alkene gives the product shown after...Ch. 6 - Prob. 95PCh. 6 - Prob. 96PCh. 6 - Prob. 97PCh. 6 - Prob. 98PCh. 6 - Prob. 99PCh. 6 - Prob. 100PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 102PCh. 6 - Prob. 103P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- State the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.arrow_forwardWhy are normal electrode potentials also called relative electrode potentials?arrow_forwardEasily differentiate between electrochemical potential and Galvani potential.arrow_forward
- Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forwardplease help with hwarrow_forwardhelp me solve this hwarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License