
(a)
Interpretation:
It should be identified that the reaction between
Concept introduction:
Regioselective: The reaction is considered as regioselective if it gives rise to specific constitutional isomer.
Constitutional Isomers: Two compounds are considered as constitutional isomers if they have same molecular formula but different in their connectivity.
Addition Reaction: It is defined as
In addition reaction of
Carbocation: it is carbon ion that bears a positive charge on it.
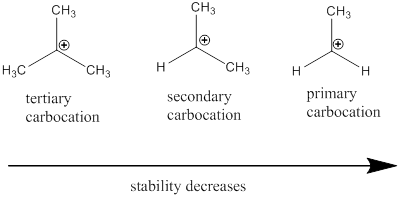
Carbocation stability order:
(b)
Interpretation:
It should be identified that the reaction between
Concept introduction:
Stereo selective: The reaction is considered as stereo selective if it gives rise to only specific stereo isomer.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Geometric isomers: Two compounds are considered as geometric isomers of each other if both contains same number of atoms but different in their arrangement that is compound is regarded as cis if identical substituents are placed on same side and trans if they are placed on the opposite sides.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Erythro product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on the same side.
Threo product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on opposite side.
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(c)
Interpretation:
It should be identified that the reaction between
Concept introduction:
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Geometric isomers: Two compounds are considered as geometric isomers of each other if both contains same number of atoms but different in their arrangement that is compound is regarded as cis if identical substituents are placed on same side and trans if they are placed on the opposite sides.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Erythro product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on the same side.
Threo product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on opposite side.
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(d)
Interpretation:
It should be identified that the reaction between
Concept introduction:
Regioselective: The reaction is considered as regioselective if it gives rise to specific constitutional isomer.
Geometric isomers: Two compounds are considered as geometric isomers of each other if both contains same number of atoms but different in their arrangement that is compound is regarded as cis if identical substituents are placed on same side and trans if they are placed on the opposite sides.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Erythro product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on the same side.
Threo product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on opposite side.
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(e)
Interpretation:
It should be identified that the reaction between
Concept introduction:
Stereo selective: The reaction is considered as stereo selective if it gives rise to only specific stereo isomer.
Geometric isomers: Two compounds are considered as geometric isomers of each other if both contains same number of atoms but different in their arrangement that is compound is regarded as cis if identical substituents are placed on same side and trans if they are placed on the opposite sides.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Erythro product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on the same side.
Threo product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on opposite side.
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(f)
Interpretation:
It should be identified that the reaction between
Concept introduction:
Stereo specific: The reaction is considered as stereo specific if the reactant is stereo isomers that give rise to different set of stereo isomers.
Geometric isomers: Two compounds are considered as geometric isomers of each other if both contains same number of atoms but different in their arrangement that is compound is regarded as cis if identical substituents are placed on same side and trans if they are placed on the opposite sides.
E configuration: The geometric isomers are given E configuration if high priority groups are placed on opposite sides of the bond.
Z configuration: The geometric isomers are given Z configuration if high priority groups are placed on same sides of the bond.
Erythro product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on the same side.
Threo product: It is the representation of carbohydrates in Fischer projection when two same substituents are placed on opposite side.
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





