
Concept explainers
(a)
Interpretation:
The mechanism for the given reaction should be proposed.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
(b)
Interpretation:
The type of carbo cation formed first should be determined.
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
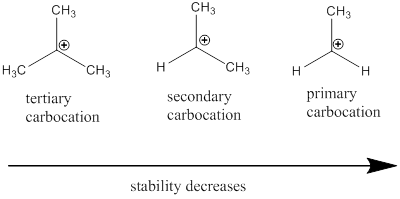
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
(c)
Interpretation:
The type of carbo cation formed after rearrangement should be determined.
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(d)
Interpretation:
The reason behind the rearrangement should be determined.
Concept introduction:
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
- Ordene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forward
- Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
