
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 61P
What are the products of the following reactions? Indicate whether each reaction is an oxidation or a reduction.
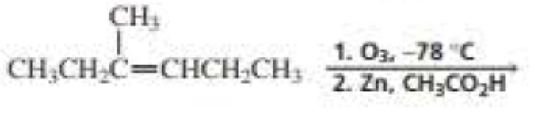
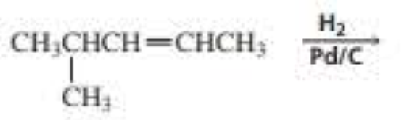
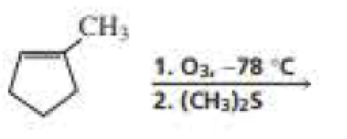
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In the electrode Pt, H2(1 atm) | H+(a=1) at 298K is 0.79 mA cm-2. If the balance potential of the electrode is -0.118 V and the potential difference of the interface is +5 mV. Determine its potential.
In one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.
In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are avilable for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.4 - Prob. 6PCh. 6.4 - Prob. 7PCh. 6.4 - What alkene should be used to synthesize each of...Ch. 6.5 - The pKa of a protonated alcohol is about 2.5, and...Ch. 6.5 - Prob. 10P
Ch. 6.5 - Prob. 11PCh. 6.6 - a. What is the major product or each or the...Ch. 6.6 - Prob. 14PCh. 6.6 - Prob. 15PCh. 6.7 - What is the major product obtained from the...Ch. 6.8 - Which is more highly regionselective: reaction of...Ch. 6.8 - Prob. 19PCh. 6.9 - What will be the product of the preceding reaction...Ch. 6.9 - Prob. 21PCh. 6.9 - Prob. 22PCh. 6.9 - Prob. 23PCh. 6.9 - What is the product of the addition of 1Cl to...Ch. 6.9 - What will be the major product obtained from the...Ch. 6.9 - Propose a mechanism for the following reaction:Ch. 6.10 - Draw structures for the following: a. 24...Ch. 6.10 - What alkene would you treat with a peroxyacid in...Ch. 6.11 - What products are formed when the following...Ch. 6.11 - Prob. 31PCh. 6.11 - Prob. 32PCh. 6.11 - The following product was obtained from the...Ch. 6.12 - What characteristics must the reactant of a...Ch. 6.13 - Prob. 36PCh. 6.13 - What stereoisomers are obtained from each of the...Ch. 6.13 - Prob. 41PCh. 6.13 - Prob. 42PCh. 6.13 - Prob. 43PCh. 6.13 - Prob. 45PCh. 6.13 - Prob. 46PCh. 6.13 - Prob. 47PCh. 6.13 - Prob. 48PCh. 6.13 - Prob. 49PCh. 6.13 - Prob. 50PCh. 6.14 - Prob. 51PCh. 6.16 - Prob. 53PCh. 6.16 - Explain why 3-methykyclohexene should not be used...Ch. 6 - Prob. 55PCh. 6 - Prob. 56PCh. 6 - Prob. 57PCh. 6 - What is the major product of the reaction of...Ch. 6 - Give two names for each of the following:Ch. 6 - Prob. 60PCh. 6 - What are the products of the following reactions?...Ch. 6 - When 3-methyl-1-butene reacts with HBr, two alkyl...Ch. 6 - Draw curved arrows to show the flow of electrons...Ch. 6 - What reagents are needed to carry out the...Ch. 6 - Prob. 65PCh. 6 - Prob. 66PCh. 6 - Prob. 67PCh. 6 - What is more stable? a. CH3C+HCH3orCH3C+HCH2ClCh. 6 - Prob. 69PCh. 6 - a. Draw the product or products that will be...Ch. 6 - Prob. 71PCh. 6 - The second-order rate constant (in units of M1s1)...Ch. 6 - Which compound has the greater dipole moment?Ch. 6 - Prob. 74PCh. 6 - Prob. 75PCh. 6 - Prob. 76PCh. 6 - Prob. 77PCh. 6 - Prob. 78PCh. 6 - Prob. 79PCh. 6 - Prob. 80PCh. 6 - Prob. 81PCh. 6 - Prob. 82PCh. 6 - Prob. 83PCh. 6 - Prob. 84PCh. 6 - Prob. 85PCh. 6 - Prob. 86PCh. 6 - Draw the products of the following reactions. If...Ch. 6 - Prob. 88PCh. 6 - Prob. 89PCh. 6 - Prob. 90PCh. 6 - Two chemists at Dupont found that lCH2Znl is...Ch. 6 - Prob. 92PCh. 6 - Prob. 93PCh. 6 - What alkene gives the product shown after...Ch. 6 - Prob. 95PCh. 6 - Prob. 96PCh. 6 - Prob. 97PCh. 6 - Prob. 98PCh. 6 - Prob. 99PCh. 6 - Prob. 100PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 102PCh. 6 - Prob. 103P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forwardIf the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forward
- Two cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forwardWith the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forwardApply the NANSTE law to the MnO4- + 8H+ + 5e- ⇄ Mn2+ + 4H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY