
(a)
Interpretation:
The alkene required to produce the product with set of conditions that is
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
The curved arrows are generally used to indicate the flow of electrons present in the reaction.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
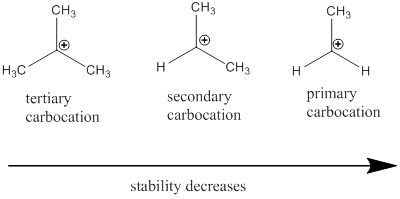
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
Ozonolysis Reaction: It is an oxidative reaction which is used to oxidize the carbon-carbon double and triple bond.
(b)
Interpretation:
The alkene required to produce the product with set of conditions that is ketone compound with three carbons with it should be identified.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
The curved arrows are generally used to indicate the flow of electrons present in the reaction.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Ozonolysis Reaction: It is an oxidative reaction which is used to oxidize the carbon-carbon double and triple bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
- Indicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forwardIf I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

