
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6.11, Problem 34P
The following product was obtained from the ozonolysis of an
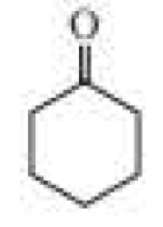
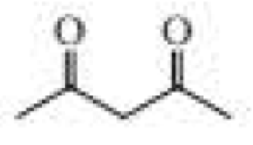
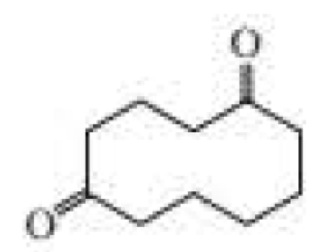
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How to name hydrocarbons
Please do these questions within the SCH4U course please with full steps since I am still unsure how to format my answers! Thank you so much.
When two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are avilable for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.4 - Prob. 6PCh. 6.4 - Prob. 7PCh. 6.4 - What alkene should be used to synthesize each of...Ch. 6.5 - The pKa of a protonated alcohol is about 2.5, and...Ch. 6.5 - Prob. 10P
Ch. 6.5 - Prob. 11PCh. 6.6 - a. What is the major product or each or the...Ch. 6.6 - Prob. 14PCh. 6.6 - Prob. 15PCh. 6.7 - What is the major product obtained from the...Ch. 6.8 - Which is more highly regionselective: reaction of...Ch. 6.8 - Prob. 19PCh. 6.9 - What will be the product of the preceding reaction...Ch. 6.9 - Prob. 21PCh. 6.9 - Prob. 22PCh. 6.9 - Prob. 23PCh. 6.9 - What is the product of the addition of 1Cl to...Ch. 6.9 - What will be the major product obtained from the...Ch. 6.9 - Propose a mechanism for the following reaction:Ch. 6.10 - Draw structures for the following: a. 24...Ch. 6.10 - What alkene would you treat with a peroxyacid in...Ch. 6.11 - What products are formed when the following...Ch. 6.11 - Prob. 31PCh. 6.11 - Prob. 32PCh. 6.11 - The following product was obtained from the...Ch. 6.12 - What characteristics must the reactant of a...Ch. 6.13 - Prob. 36PCh. 6.13 - What stereoisomers are obtained from each of the...Ch. 6.13 - Prob. 41PCh. 6.13 - Prob. 42PCh. 6.13 - Prob. 43PCh. 6.13 - Prob. 45PCh. 6.13 - Prob. 46PCh. 6.13 - Prob. 47PCh. 6.13 - Prob. 48PCh. 6.13 - Prob. 49PCh. 6.13 - Prob. 50PCh. 6.14 - Prob. 51PCh. 6.16 - Prob. 53PCh. 6.16 - Explain why 3-methykyclohexene should not be used...Ch. 6 - Prob. 55PCh. 6 - Prob. 56PCh. 6 - Prob. 57PCh. 6 - What is the major product of the reaction of...Ch. 6 - Give two names for each of the following:Ch. 6 - Prob. 60PCh. 6 - What are the products of the following reactions?...Ch. 6 - When 3-methyl-1-butene reacts with HBr, two alkyl...Ch. 6 - Draw curved arrows to show the flow of electrons...Ch. 6 - What reagents are needed to carry out the...Ch. 6 - Prob. 65PCh. 6 - Prob. 66PCh. 6 - Prob. 67PCh. 6 - What is more stable? a. CH3C+HCH3orCH3C+HCH2ClCh. 6 - Prob. 69PCh. 6 - a. Draw the product or products that will be...Ch. 6 - Prob. 71PCh. 6 - The second-order rate constant (in units of M1s1)...Ch. 6 - Which compound has the greater dipole moment?Ch. 6 - Prob. 74PCh. 6 - Prob. 75PCh. 6 - Prob. 76PCh. 6 - Prob. 77PCh. 6 - Prob. 78PCh. 6 - Prob. 79PCh. 6 - Prob. 80PCh. 6 - Prob. 81PCh. 6 - Prob. 82PCh. 6 - Prob. 83PCh. 6 - Prob. 84PCh. 6 - Prob. 85PCh. 6 - Prob. 86PCh. 6 - Draw the products of the following reactions. If...Ch. 6 - Prob. 88PCh. 6 - Prob. 89PCh. 6 - Prob. 90PCh. 6 - Two chemists at Dupont found that lCH2Znl is...Ch. 6 - Prob. 92PCh. 6 - Prob. 93PCh. 6 - What alkene gives the product shown after...Ch. 6 - Prob. 95PCh. 6 - Prob. 96PCh. 6 - Prob. 97PCh. 6 - Prob. 98PCh. 6 - Prob. 99PCh. 6 - Prob. 100PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 102PCh. 6 - Prob. 103P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.arrow_forwardGiven the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.arrow_forwardThe decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward
- 2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forwardThe emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forwardIndicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forward
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Seven Name Reactions in One - Palladium Catalysed Reaction (047 - 053); Author: Rasayan Academy - Jagriti Sharma;https://www.youtube.com/watch?v=5HEKTpDFkqI;License: Standard YouTube License, CC-BY