
(a)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Reduction Reaction: It is just opposite of oxidation reaction which involves removal of oxygen atoms or addition of hydrogen atoms and addition of electrons.
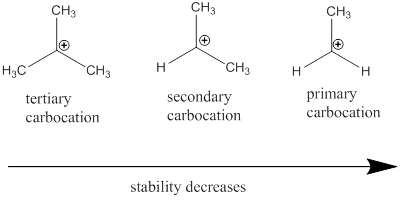
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
(b)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Addition of halogen to an
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(c)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(d)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(e)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
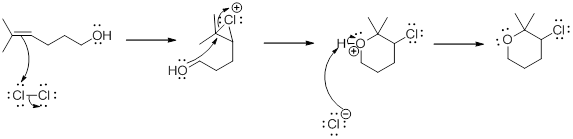
Addition of halogen to an alkene: The addition of halogen to an alkene compound forms cyclic 3 membered intermediate as the first step which then the leads to the product formation. Example for this is as follows,

Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(f)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(g)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Acid Catalyzed Hydration Reaction: The reaction involves breaking of phi bonds between carbon-carbon multiple bonds and addition of alcohol to more substituted position of carbon in the molecule.
First step is the acid donates proton to the alkene which leads to the formation of more stable carbo cation.
Then, the water is added to the given alkene through acid catalyzed reaction where the water gets added to the carbo cation finally, the removal of one proton from oxonium ion (oxygen with one positive charge) using water results in the formation of product.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

(h)
Interpretation:
The major product for the given reaction should be determined.
Concept introduction:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Electrophilic addition: It is a type of addition reaction in which the pi bond present in the molecule breaks as the electrophile approaches and results in the formation of product with sigma bond.
Addition of halogen to an alkene: The addition of halogen to an alkene compound forms cyclic 3 membered intermediate as the first step which then the leads to the product formation. Example for this is as follows,

Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
