
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.6, Problem 4.44P
Predict the products for each of the following reactions:
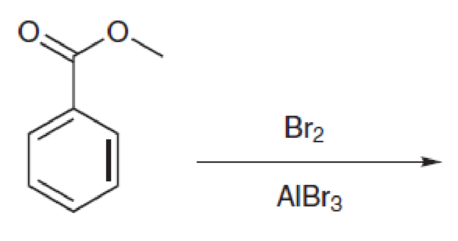
Hint: The group on the ring is a deactivator.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a mechanism to account for the formation of each
NaOH
product in the following reaction. Hint: Under these
conditions, deprotonation of a propargylic (C=C-CH)
A
D
Br
carbon is reversible.
Draw the two final products for the following reaction. Watch the stereochemistry!
H,O,
NMe,
NMo,
Ph
H,
Ph
H,
Hint: An elimination reactions occurs in the next step. What is the leaving group?
please help with this question. thank you.
How could you synthesize the following compound via a crossed aldol condensation? Hint: an a,b-unsaturated ketone can undergo catalytic hydrogenation to yield a saturated ketone.
Chapter 4 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 4.1 - Consider the following reaction, in which an...Ch. 4.1 - Prob. 4.3PCh. 4.1 - Aromatic rings will also undergo iodination when...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.3 - Prob. 4.10PCh. 4.3 - Prob. 4.11PCh. 4.3 - Prob. 4.12PCh. 4.3 - Prob. 4.13P
Ch. 4.3 - Prob. 4.14PCh. 4.3 - Predict the products of the following reaction.Ch. 4.3 - Prob. 4.16PCh. 4.3 - Prob. 4.17PCh. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - Prob. 4.27PCh. 4.4 - Prob. 4.28PCh. 4.4 - And now, for a challenging problem, try to draw...Ch. 4.6 - Prob. 4.31PCh. 4.6 - Prob. 4.32PCh. 4.6 - Prob. 4.33PCh. 4.6 - Prob. 4.34PCh. 4.6 - Prob. 4.35PCh. 4.6 - Prob. 4.36PCh. 4.6 - Prob. 4.37PCh. 4.6 - Prob. 4.40PCh. 4.6 - Prob. 4.41PCh. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Prob. 4.47PCh. 4.6 - Prob. 4.48PCh. 4.6 - Prob. 4.49PCh. 4.6 - Prob. 4.50PCh. 4.6 - Prob. 4.51PCh. 4.6 - Prob. 4.52PCh. 4.6 - Prob. 4.53PCh. 4.6 - Prob. 4.54PCh. 4.6 - Prob. 4.55PCh. 4.6 - Prob. 4.56PCh. 4.7 - Prob. 4.58PCh. 4.7 - Prob. 4.59PCh. 4.7 - Prob. 4.60PCh. 4.7 - Prob. 4.61PCh. 4.7 - Prob. 4.62PCh. 4.7 - Prob. 4.63PCh. 4.7 - Prob. 4.64PCh. 4.7 - Prob. 4.65PCh. 4.7 - Prob. 4.66PCh. 4.7 - Prob. 4.67PCh. 4.7 - Can you explain why the following group is a...Ch. 4.7 - Prob. 4.70PCh. 4.7 - Prob. 4.71PCh. 4.7 - Prob. 4.72PCh. 4.7 - Prob. 4.73PCh. 4.7 - Prob. 4.74PCh. 4.7 - Prob. 4.76PCh. 4.7 - Prob. 4.77PCh. 4.7 - Prob. 4.78PCh. 4.7 - Prob. 4.79PCh. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Prob. 4.87PCh. 4.8 - Prob. 4.88PCh. 4.8 - Prob. 4.89PCh. 4.8 - Prob. 4.90PCh. 4.8 - Prob. 4.91PCh. 4.8 - Prob. 4.92PCh. 4.9 - Prob. 4.94PCh. 4.9 - Prob. 4.95PCh. 4.9 - Prob. 4.96PCh. 4.9 - Prob. 4.97PCh. 4.9 - Prob. 4.98PCh. 4.9 - Prob. 4.99PCh. 4.9 - Prob. 4.100PCh. 4.9 - Prob. 4.101PCh. 4.9 - Prob. 4.102P
Additional Science Textbook Solutions
Find more solutions based on key concepts
11.57 Draw the cis and trans isomers for each of the following: (11.6)
a. 2-pentene
b. 3-hexene
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
39. Consider the reaction:
Kp = 28.4 at 298 K
In a reaction mixture at equilibrium, the partial pressure...
Chemistry: Structure and Properties
7.141 Use the periodic table to select the element in the following list for which there is the largest differe...
Chemistry: The Molecular Nature of Matter
The conclusion is given for two substances from given heat of fusion of water and ethanol. Concept introduction...
Living By Chemistry: First Edition Textbook
Without looking at the structures, give molecular formulas for the compounds in Problem 3-8 (a) and (b). Use th...
Organic Chemistry (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the presence of a base which of the following will not form an enolate?arrow_forwardDraw the mechanism of the following alkylation reaction that occurs via an enolate intermediate. о сн, о сн, (1) LDA Ph-C-CH-CH, Ph-C-C-CH, (2) Ph — CH, — Вr ČH,-Pharrow_forwardDraw the major organic product of the reaction shown.arrow_forward
- When benzaldehyde is treated with a catalytic amount of KCN, the benzoin condensation occurs. Draw a complete, detailed mechanism for this reaction. Hint: The reaction does not take place without the presence of NC¯. An a-hydroxy ketone ОН KCN HOH,0, ELOH 2-Hydroxy-1,2-diphenylethanone (Benzoin) 88% Benzaldehydearrow_forward18en the folbwng sequence of reac ond what wo structure of compound AT D (CH,,CULI 2) H,0 HN-CH, H,0 Select one 2.Wnchufthfollewine.comectly represert eet the stepm the rechnsnetamad altalcardensation reactarrow_forwardPlease answer both questionsarrow_forward
- uld you proceed with the following synthesis Include intermediate comp formed. 3. What is the main major product(s) of the following reaction. Draw a mechanism with accompanying resonance structures to explain your answer. OMe Br₂ FeBr3arrow_forwardPlease answer it all necessary stepsarrow_forwardDraw a detailed mechanism for the following reaction and include the major productarrow_forward
- Draw a mechanism for the following reactions "NO, NaOH, Н,О heat 1) NaOH 2) NaOH, heat O.arrow_forwardDraw a detailed mechanism and show the product for the following reaction. HO H₂SO4arrow_forwardWhen an electrophilic aromatic substitution reaction on naphthalene is reversible, such as in a sulfonation reaction, the major product is the one that is most stable. With this in mind, predict the major product of the reaction shown here.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY