
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.2, Problem 4.5P
In each of the following cases, identify the reagents that you would use in order to achieve the desired transformation.
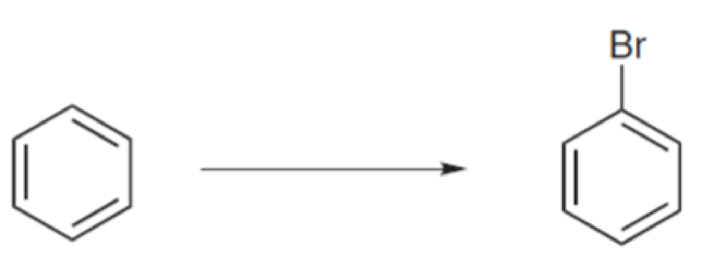
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the appropriate reagent(s) for the following transformation
For each of the transformations given below, draw the structures of the named starting
material, products and possible intermediates. State the necessary reagents and conditions
in the reaction scheme. More than one step may be required.
cyclohexylmethanol
ethenylbenzene
pent-1-ene
2-methylpentan-3-ol
cyclohexanecarbaldehyde
acetophenone
2-methylpenta-2-amine
propanoic acid + propanone
a)
b)
In the provided boxes, provide synthetic sequences (including reagents used and the compound produced in each intermediate step) for the following transformations.
Chapter 4 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 4.1 - Consider the following reaction, in which an...Ch. 4.1 - Prob. 4.3PCh. 4.1 - Aromatic rings will also undergo iodination when...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.3 - Prob. 4.10PCh. 4.3 - Prob. 4.11PCh. 4.3 - Prob. 4.12PCh. 4.3 - Prob. 4.13P
Ch. 4.3 - Prob. 4.14PCh. 4.3 - Predict the products of the following reaction.Ch. 4.3 - Prob. 4.16PCh. 4.3 - Prob. 4.17PCh. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - Prob. 4.27PCh. 4.4 - Prob. 4.28PCh. 4.4 - And now, for a challenging problem, try to draw...Ch. 4.6 - Prob. 4.31PCh. 4.6 - Prob. 4.32PCh. 4.6 - Prob. 4.33PCh. 4.6 - Prob. 4.34PCh. 4.6 - Prob. 4.35PCh. 4.6 - Prob. 4.36PCh. 4.6 - Prob. 4.37PCh. 4.6 - Prob. 4.40PCh. 4.6 - Prob. 4.41PCh. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Prob. 4.47PCh. 4.6 - Prob. 4.48PCh. 4.6 - Prob. 4.49PCh. 4.6 - Prob. 4.50PCh. 4.6 - Prob. 4.51PCh. 4.6 - Prob. 4.52PCh. 4.6 - Prob. 4.53PCh. 4.6 - Prob. 4.54PCh. 4.6 - Prob. 4.55PCh. 4.6 - Prob. 4.56PCh. 4.7 - Prob. 4.58PCh. 4.7 - Prob. 4.59PCh. 4.7 - Prob. 4.60PCh. 4.7 - Prob. 4.61PCh. 4.7 - Prob. 4.62PCh. 4.7 - Prob. 4.63PCh. 4.7 - Prob. 4.64PCh. 4.7 - Prob. 4.65PCh. 4.7 - Prob. 4.66PCh. 4.7 - Prob. 4.67PCh. 4.7 - Can you explain why the following group is a...Ch. 4.7 - Prob. 4.70PCh. 4.7 - Prob. 4.71PCh. 4.7 - Prob. 4.72PCh. 4.7 - Prob. 4.73PCh. 4.7 - Prob. 4.74PCh. 4.7 - Prob. 4.76PCh. 4.7 - Prob. 4.77PCh. 4.7 - Prob. 4.78PCh. 4.7 - Prob. 4.79PCh. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Prob. 4.87PCh. 4.8 - Prob. 4.88PCh. 4.8 - Prob. 4.89PCh. 4.8 - Prob. 4.90PCh. 4.8 - Prob. 4.91PCh. 4.8 - Prob. 4.92PCh. 4.9 - Prob. 4.94PCh. 4.9 - Prob. 4.95PCh. 4.9 - Prob. 4.96PCh. 4.9 - Prob. 4.97PCh. 4.9 - Prob. 4.98PCh. 4.9 - Prob. 4.99PCh. 4.9 - Prob. 4.100PCh. 4.9 - Prob. 4.101PCh. 4.9 - Prob. 4.102P
Additional Science Textbook Solutions
Find more solutions based on key concepts
110. Have each group member choose a set of quantum numbers for an electron in a hydrogen atom. Calculate the w...
Chemistry: Structure and Properties (2nd Edition)
Practice Problem 2.26
Which compound would you expect to have the higher melting point, propane or cyclo-propan...
Organic Chemistry
1. What is thermochemistry? Why is it important?
Chemistry: Structure and Properties
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
23. Give the symbol and name for (a) an isotope with a mass number of 37 and an atomic number of 17 and (b) an ...
Chemistry For Changing Times (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 1-bromobutane. First, select the reagents necessary for the transformation shown, using no more than four steps in the synthesis. In part 2, you will synthesize any carbon reagents (other than acetylene) that were used in the synthesis. The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer.arrow_forwardIn the provided boxes, provide synthetic sequences (including reagents used and the compound produced in each intermediate step) for the following transformations.arrow_forwardProvide all reagents, material, and solvents needed to complete the following transformations. Any reagent you add cannot be more than 5 carbons, except benzene and reagents provided in the problem.arrow_forward
- As we will learn in Chapter 9, an epoxide is an ether with an oxygen atom in a three-membered ring. Epoxides can be made by intramolecular SN2 reactions of intermediates that contain a nucleophile and a leaving group on adjacent carbons, as shown.Assume that each of the following starting materials can be converted to an epoxide by this reaction. Draw the product formed (including stereochemistry) from each starting material. Why might some of these reactions be more difficult than others in yielding nucleophilic substitution products?arrow_forwardIn the provided boxes, provide synthetic sequences (including reagents used and the compound produced in each intermediate step) for the following transformations. HO NH₂ i NH₂arrow_forwardStarting with benzene and using any other necessary reagents of your choice, create a synthesis (or sytheses) for each of the following compounds.arrow_forward
- Suggest possible synthetic route for each of following transformations. Show the reagents,intermediates and products.arrow_forwardPropose a series of reactions that would convert the provided starting material into the given product. The synthesis will take more than one step. For each reaction you use, show the reagents you would use and the product of that step. In each step, your desired product should be formed as the major product. Assume chiral products are formed as racemic mixtures. Your reactions should give the correct diastereomer. In addition to the provided starting material, you may use any other organic compound as well as any reagents we have discussed. Could someone please help me with this practice problem? Thank you!arrow_forwardShow how you would accomplish the following synthetic conversion. More than one step may be required.arrow_forward
- All rearrangements we have discussed so far have involved generation of an electron-deficient carbon followed by a 1,2-shift of an atom or a group of atoms from an adjacent atom to the electron-deficient carbon. Rearrangements by a 1,2-shift can also occur following the generation of an electron-deficient oxygen. Propose a mechanism for the acid-catalyzed rearrangement of cumene hydroperoxide to phenol and acetone.arrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forwardProvide the reagent necessary and mechanism for the transformation below (more than one step is required). Additionally provide the IUPAC name for the starting materialarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY