
Interpretation:
The balanced chemical equations for photosynthesis,
Concept introduction:
Plants make their food by the process of photosynthesis. Cellular respiration is a redox process.
Answer to Problem 92A
The balanced chemical equation for photosynthesis is,
The balanced chemical equation for cellular respiration is,
The balanced chemical equation for the hydrolysis of lactose is,
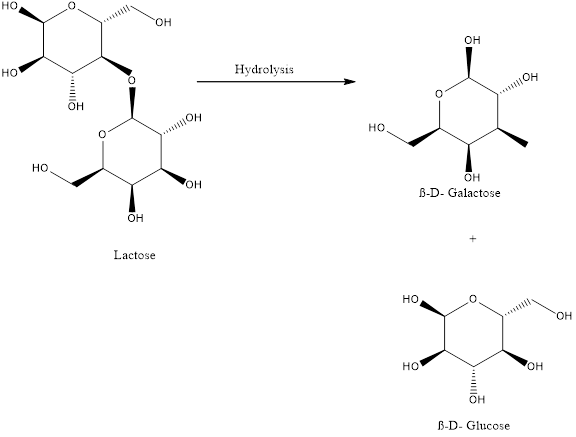
Explanation of Solution
The balanced chemical equation for photosynthesis is,
Carbon dioxide reacts with water in presence of light to give glucose and oxygen.
The balanced chemical equation for cellular respiration is,
Glucose reacts with oxygen to give carbon dioxide, water and energy.
The balanced chemical equation for the hydrolysis of lactose is,
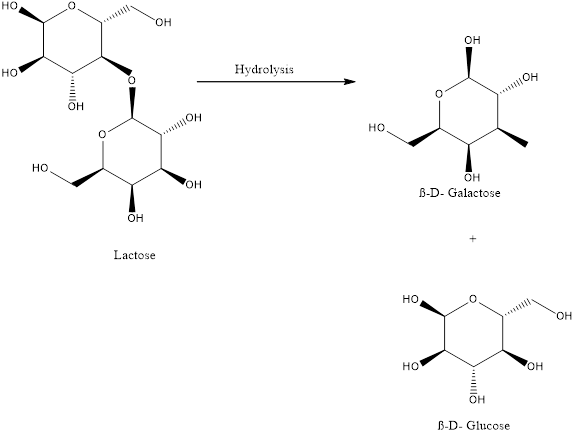
Lactose undergoes hydrolysis to give D Galactose and D Glucose.
Carbon dioxide reacts with water in presence of light to give glucose and oxygen. Glucose reacts with oxygen to give carbon dioxide, water and energy and Lactose undergoes hydrolysis to give D Galactose and D Glucose..
Chapter 23 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Biological Science (6th Edition)
Cosmic Perspective Fundamentals
Biology: Life on Earth (11th Edition)
Introductory Chemistry (6th Edition)
Microbiology with Diseases by Body System (5th Edition)
College Physics: A Strategic Approach (3rd Edition)
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





