
Interpretation:
The reason should be determined for the following statement along with an equation.
Disaccharides and polysaccharides can't be broken down in the absence of water.
Concept introduction:
A biological molecule which consists of carbon, hydrogen and oxygen is known as saccharide or carbohydrate. The general formula of saccharide is
Saccharides (sugar) are classified as: monosaccharides, disaccharides and polysaccharides.
Monosaccharides are defined as a simpler carbohydrate which contains one sugar molecule and can’t ne hydrolysed into smaller carbohydrate.
Disaccharides are defined as a saccharide which contains two sugar molecules that is when two monosaccharides are linked by glycosidic bonds. It is a double ring structure.
Polysaccharides are defined as a saccharide which contains many units of sugar molecules that is more than two monosaccharides are linked by glycosidic bonds.
Explanation of Solution
Disaccharides are made up of two monosaccharide units. When two monosaccharides react or combined with each other, results in the formation of a disaccharide with the removal of water and the bond formed between them is ether or glycosidic bond. The reaction is known as condensation reaction.
Now, in the reverse process, a disaccharide digested to form two monosaccharides in the presence of water with the breaking of ether bond at same time. The reaction is known as hydrolysis reaction.
For an example:
The hydrolysis of sucrose results in the formation of glucose and fructose.
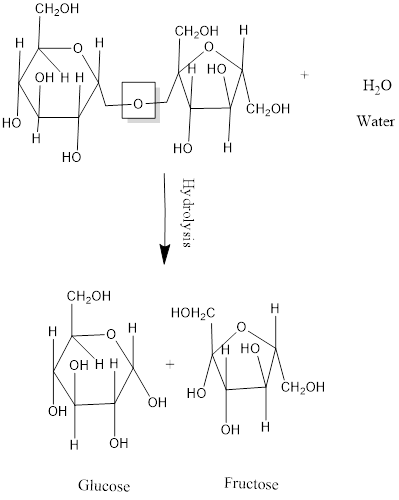
Thus, addition of water molecule is necessary to break the ether bond or glycosidic bond present between the disaccharide.
Chapter 23 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Chemistry: The Central Science (14th Edition)
Introductory Chemistry (6th Edition)
Cosmic Perspective Fundamentals
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Can u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
- Explain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forwardExplain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





