
(a)
Interpretation: The difference in basicity between ortho- or para- and meta-nitroanilines has to be discussed
Concept Introduction:
Resonance is a mental exercise and method within the
To find: para-nitroaniline is an order of magnitude less basic than meta-nitroaniline. Find the reason for the given statement
Find the delocalization behavior of para-nitroaniline
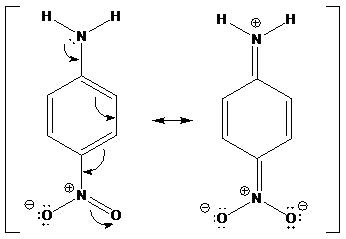
(b)
Interpretation: The difference in basicity between ortho- or para- and meta-nitroanilines has to be discussed
Concept Introduction:
Resonance is a mental exercise and method within the Valence Bond Theory of bonding that describes the delocalization of electrons within molecules. The net sum of valid resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. A molecule that has several resonance structures is more stable than one with fewer. If the delocalization of electrons within molecules is more, the compound is less basic and vice versa.
To find: Finding the basicity of ortho-nitroaniline which is closer in value to meta- or to para-nitroaniline
Find the delocalization behavior of ortho-nitroaniline

Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- Speaking of composite materials, indicate the correct option:(A). Composite materials can only be: metal-polymer or polymer-polymer.(B). Composite materials can be made up of particles, but not fibers or sheets.(C). When the reinforcing particles are uniformly distributed in a composite material, there may be a greater tendency for it to have isotropic properties.(D). None of the above is correct.arrow_forwardIf we are talking about viscoelastic modulus or viscoelastic relaxation modulus in polymers, indicate the correct option.(A). It reports the variation of elastic behavior as a function of time.(B). It is only useful for defining its glass transition temperature.(C). It only allows us to define the polymer degradation temperature.(D). Neither option is correct.arrow_forwardWhen natural light falls perpendicularly on a material A, it has a reflectivity of 0.813%. Indicate the value of the refractive index.arrow_forward
- In piezoelectricity and piezoelectric ceramics, one of the following options is false:(A). Piezoelectricity allows an electrical signal to be transformed into a mechanical one.(B). PbZrO3 is a well-known piezoelectric ceramic.(C). Piezoelectricity and ferroelectricity in general have no relationship.(D). One of the applications of piezoelectricity is sonar.arrow_forward(30 MARKS) Give the major product(s ) formed including relevant stereochemistry or the complete reaction conditions for the following reactions. More than one step may be required for each reaction arrow, in which case the steps must be numbered 1), 2) etc. (2 marks each box) h) i) h) OH i) HO H3PO4, heat 2 Brarrow_forwardNonearrow_forward
- Indicate which option is false(A). Resistivity has a residual component and a thermal component.(B). In some materials resistivity increases with T and in others it decreases.(C). In insulating materials, resistivity is very low.arrow_forwardIn ceramic materials, in relation to polymorphism, the same substance crystallizes differently when external conditions vary. Is this correct?arrow_forwardIndicate the type of bond that is considered to be a hydrogen bond.(A). Permanent dipole-dipole interaction between polar molecules.(B). Mixed ionic-covalent bond.(C). Principal interatomic bond(D). Van del Waals forces.arrow_forward
- Retro aldol: NaOH H₂O H NaOH & d H₂O Harrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. H conc. HBr Drawing Qarrow_forwardCalculate the atomic packing factor of diamond knowing that the number of Si atoms per cm3 is 2.66·1022 and that the atomic radii of silicon and oxygen are, respectively, 0.038 and 0.117 nm.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





