
(a)
Interpretation: Using a Gabriel synthesis, a give set of primary amine compounds have to be synthesized.
Concept Introduction: The general formula for primary amine is –NH2. There are several methods available to prepare primary
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
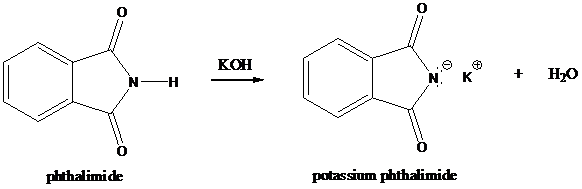
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary
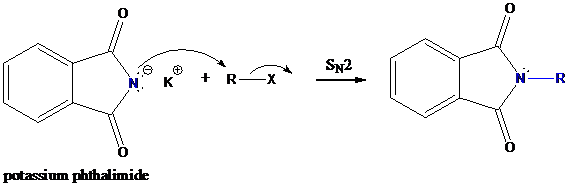
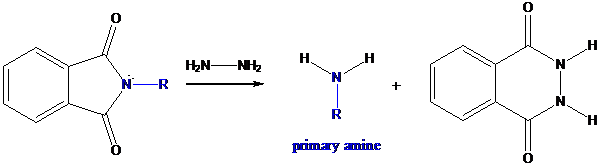
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.
(b)
Interpretation: Using a Gabriel synthesis, a give set of primary amine compounds have to be synthesized.
Concept Introduction: The general formula for primary amine is –NH2. There are several methods available to prepare primary amines. Among them, Gabriel synthesis plays a very important role for preparing it. In this method, secondary and tertiary amines are not formed as side products. It involves in three steps.
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
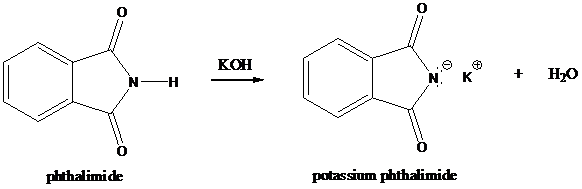
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary alkyl halides (R−X), R and X get positive and negative charges, respectively when they ionize. As a result, a bond between nitrogen of phthalimide and carbon of R is formed. This is SN2 nucleophilic substitution reaction. Halogen atom is going away as halide anion.
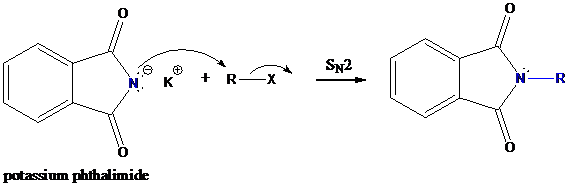
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.

(c)
Interpretation: Using a Gabriel synthesis, a give set of primary amine compounds have to be synthesized.
Concept Introduction: The general formula for primary amine is –NH2. There are several methods available to prepare primary amines. Among them, Gabriel synthesis plays a very important role for preparing it. In this method, secondary and tertiary amines are not formed as side products. It involves in three steps.
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
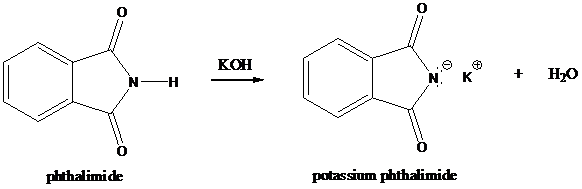
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary alkyl halides (R−X), R and X get positive and negative charges, respectively when they ionize. As a result, a bond between nitrogen of phthalimide and carbon of R is formed. This is SN2 nucleophilic substitution reaction. Halogen atom is going away as halide anion.
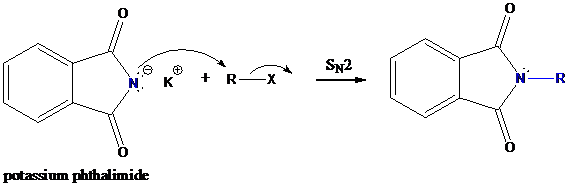
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.

(d)
Interpretation: Using a Gabriel synthesis, a give set of primary amine compounds have to be synthesized.
Concept Introduction: The general formula for primary amine is –NH2. There are several methods available to prepare primary amines. Among them, Gabriel synthesis plays a very important role for preparing it. In this method, secondary and tertiary amines are not formed as side products. It involves in three steps.
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
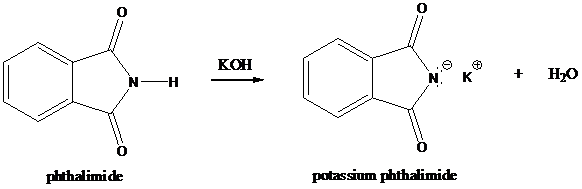
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary alkyl halides (R−X), R and X get positive and negative charges, respectively when they ionize. As a result, a bond between nitrogen of phthalimide and carbon of R is formed. This is SN2 nucleophilic substitution reaction. Halogen atom is going away as halide anion.
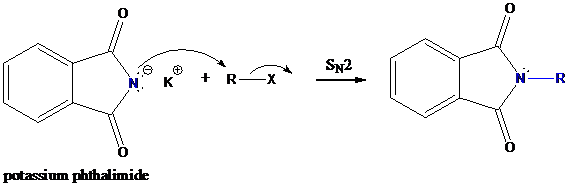
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.

Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





