
(a)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 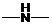 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary
groups are attached to the parent carbon, they are called primary, secondary and tertiary
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
(b)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 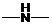 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
In the given compound (b), three alkyl groups are attached to nitrogen atom. Therefore, it belongs to tertiary amine.
Decide the given name which is in general name method or IUPAC name method
There are three alkyl groups present in the given name. Here, the main chain is cyclopropylamine. Three-membered carbon ring structure is called cyclopropyl group. So, general name system is followed.
Locate the substituents and draw the corresponding structure for the given name
(c)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 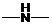 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
In the given compound (c), only one alkyl group, namely pentane is attached to nitrogen atom. Therefore, it belongs to primary amine.
Decide the given name which is in general name method or IUPAC name method
There is only one alkane group present in the given name. It is pentane attached to primary amine named as pentanamine which is the main chain of the given name. So, IUPAC name system is followed.
Locate the substituents and draw the corresponding structure for the given name
(d)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 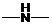 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
In the given compound (d), only one alkyl group is attached to nitrogen atom. Therefore, it belongs to primary amine.
Decide the given name which is in general name method or IUPAC name method
There is only one alkyl group present in the given name. It is benzyl group. So, simple general name system is followed. If phenyl group is attached to methylene group, it is called benzyl group.
Locate the substituents and draw the corresponding structure for the given name
Trending nowThis is a popular solution!

Chapter 22 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- Q7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forwardQ3: Rank the following compounds in increasing reactivity of E1 and E2 eliminations, respectively. Br ca. go do A CI CI B C CI Darrow_forward
- Q5: Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2). H₂O דיי "Br KN3 CH3CH2OH NaNH2 NH3 Page 3 of 6 Chem 0310 Organic Chemistry 1 HW Problem Sets CI Br excess NaOCH 3 CH3OH Br KOC(CH3)3 DuckDuckGarrow_forwardQ4: Circle the substrate that gives a single alkene product in a E2 elimination. CI CI Br Brarrow_forwardPlease calculate the chemical shift of each protonsarrow_forward
- Q1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br 'CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardQ6: Provide the reagents and conditions for the following reactions to make the product with a good yield. Br Br CI она CIarrow_forwardQ2: We would not expect the following primary alkyl halide to go through an SN1 reaction. However, it can go through an SN1 mechanism. Explain why. Hint: Think about what happens when the leaving group leaves. CI NaO EtOH H བྱིས་ Harrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





