
(a)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e)
Alkene on ozonolysis produces carbonyl compounds - f)
Alkyl halides with strong base gives alkene - g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions.
Aldehyde orketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primaryamines .
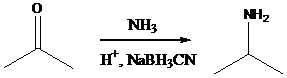
Using these concepts, we can transfer 1-hexanol into the given compounds.
(b)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e) Alkene on ozonolysis produces carbonyl compounds
- f) Alkyl halides with strong base gives alkene
- g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions. Aldehyde or ketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primary amines.
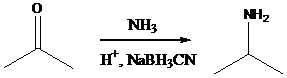
Using these concepts, we can transfer 1-hexanol into the given compounds.
(c)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e) Alkene on ozonolysis produces carbonyl compounds
- f) Alkyl halides with strong base gives alkene
- g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions. Aldehyde or ketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primary amines.
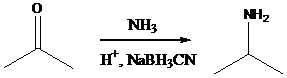
Using these concepts, we can transfer 1-hexanol into the given compounds.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- The emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation to undertand concepts.arrow_forward
- Nonearrow_forward7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forward
- Nonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward451. Use the diffusion model from lecture that showed the likelihood of mixing occurring in a lattice model with eight lattice sites: Case Left Right A B C Permeable Barrier → and show that with 2V lattice sites on each side of the permeable barrier and a total of 2V white particles and 2V black particles, that perfect de-mixing (all one color on each side of the barrier) becomes increasingly unlikely as V increases.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





