
(a)
Interpretation: An efficient synthesis for the given transformations has to be proposed.
Concept Introduction:
Friedel-Crafts Alkylation: The Friedel-Crafts alkylation involves the electrophilic substitution of alkyl groups on
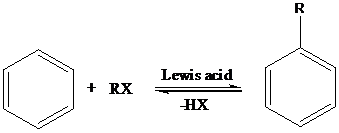
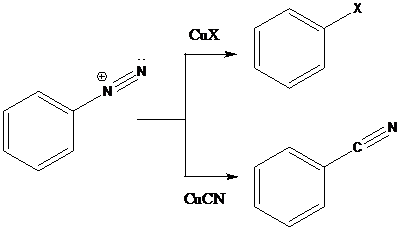
Sandmeyer reaction: Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
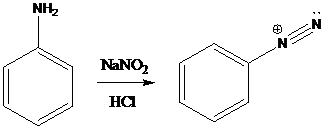
To find: Propose an efficient synthesis for the given transformation (a)
Decide the transformation
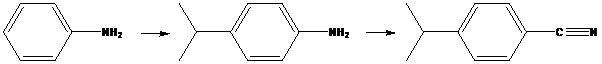
(b)
Interpretation: An efficient synthesis for the given transformations has to be proposed.
Concept Introduction:
Electrophilic aromatic substitution: Electrophilic aromatic substitution is a reaction in which the hydrogen atom of an aromatic ring is replaced as a result of an electrophilic attack on the aromatic ring. Here are three general steps to an electrophilic aromatic substitution.
- 1) Generation of an electrophile
- 2) Attack of the electrophile on the aromatic ring, creating a resonance-stabilized carbocation called an arenium ion. It loses aromaticity in this step, so the energy of activation is high. Furthermore, this is the rate-determining step of the reaction because of the disruption of aromaticity. The arenium ion is a hybrid resonance structure. There are three general resonance contributors of an arenium ion.
- 3) Deprotonation of the arenium ion by a weak base to regain aromaticity.
Sandmeyer reaction: Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.

A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
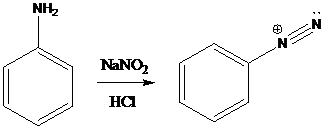
To find: Propose an efficient synthesis for the given transformation (b)
Apply a retrosynthetic analysis
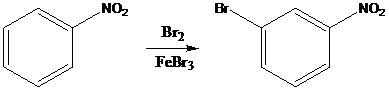
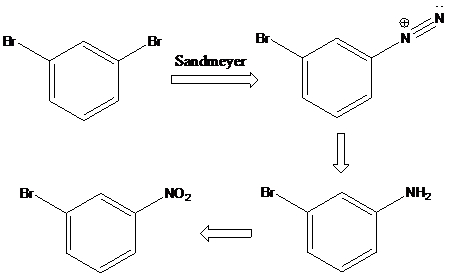
(c)
Interpretation: An efficient synthesis for the given transformations has to be proposed.
Concept Introduction:
Friedel–Crafts Acylation: The Friedel–Crafts acylation is the reaction of an arene with acyl chlorides or anhydrides using a strong Lewis acid catalyst. This reaction proceeds via electrophilic aromatic substitution to form monoacylated products.
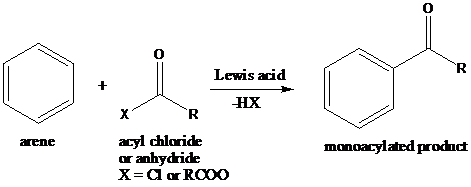
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
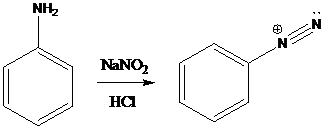
To find: Propose an efficient synthesis for the given transformation (c)
Apply a retrosynthetic analysis
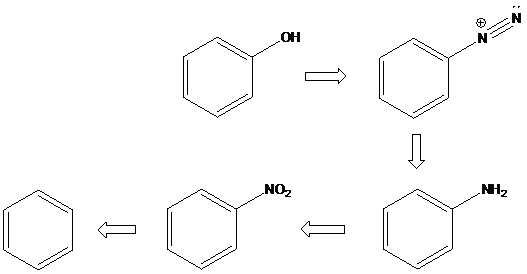
(d)
Interpretation: An efficient synthesis for the given transformations has to be proposed.
Concept Introduction:
Friedel-Crafts Alkylation: The Friedel-Crafts alkylation involves the electrophilic substitution of alkyl groups on aromatic rings when arenes are treated with alkyl halides in presence of Lewis acids. This reaction is catalyzed by Lewis acids like anhydrous AlCl3, FeX3, ZnCl2, BF3 etc.
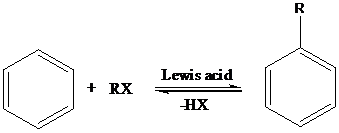
Sandmeyer reaction: Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.

A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
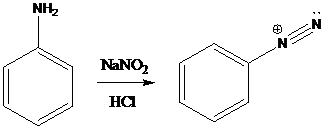
To find: Propose an efficient synthesis for the given transformation (d)
Apply a retrosynthetic analysis
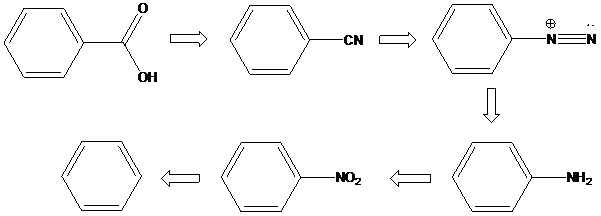
(e)
Interpretation: An efficient synthesis for the given transformations has to be proposed.
Concept Introduction:
Electrophilic aromatic substitution: Electrophilic aromatic substitution is a reaction in which the hydrogen atom of an aromatic ring is replaced as a result of an electrophilic attack on the aromatic ring. Here are three general steps to an electrophilic aromatic substitution.
- 1) Generation of an electrophile
- 2) Attack of the electrophile on the aromatic ring, creating a resonance-stabilized carbocation called an arenium ion. It loses aromaticity in this step, so the energy of activation is high. Furthermore, this is the rate-determining step of the reaction because of the disruption of aromaticity. The arenium ion is a hybrid resonance structure. There are three general resonance contributors of an arenium ion.
- 3) Deprotonation of the arenium ion by a weak base to regain aromaticity.
Schiemann reaction: It is the reaction in which diazonium salt is converted into fluoro group using fluoroboric acid (HBF4).
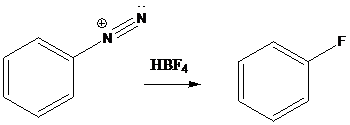
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
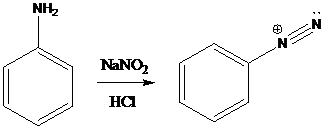
To find: Propose an efficient synthesis for the given transformation (e)
Apply a retrosynthetic analysis
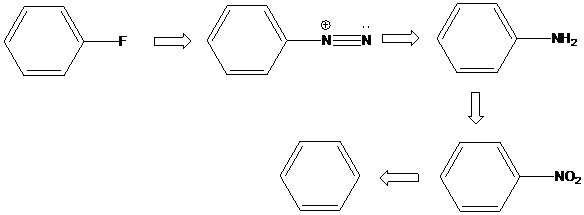
(f)
Interpretation: An efficient synthesis for the given transformations has to be proposed.
Concept Introduction:
Sandmeyer reaction: Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.

A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
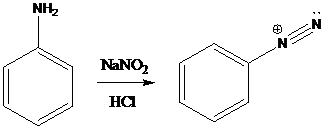
To find: Propose an efficient synthesis for the given transformation (f)
Perform elimination-addition process
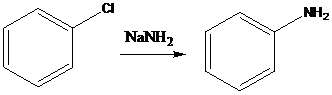
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- please provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forward
- presented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forward
- Name the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





