
Interpretation: The mechanism for the last step of Gabriel synthesis under strong basic condition has to be explained.
Concept Introduction: The general formula for hexylamine is C6H13NH2. There are several methods available to prepare primary
Step-1: Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
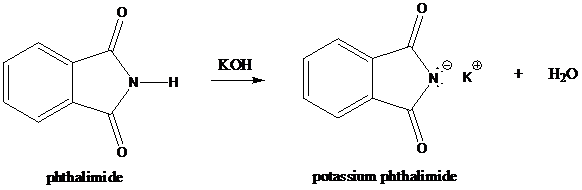
Step-2: Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary
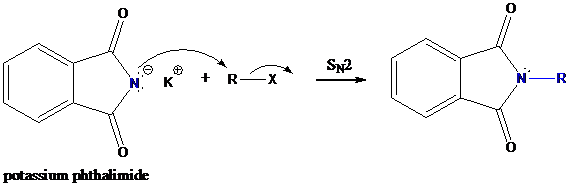
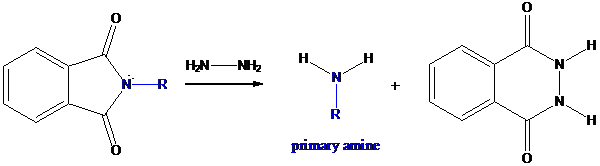
Step-3: Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- A pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forwardNonearrow_forward
- Draw the structure of the product of the reaction given the IR and MS data. Spectral analysis of the product reveals: MS: M 150, M-15, M-43 CH.COCI AICI, IR: 3150-3000 cm, 2950-2850 cm and 1700 cmarrow_forwardPart II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forwardNonearrow_forward
- Choose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





