
(a)
Interpretation:
For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 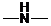 and
and 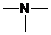 groups are attached to the parent carbon, they are called primary, secondary and tertiary
groups are attached to the parent carbon, they are called primary, secondary and tertiary
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(b)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 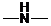 and
and 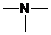 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(c)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 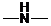 and
and 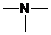 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(d)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 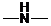 and
and 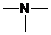 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
(e)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If ,
, 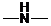 and
and 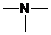 groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- Please correct answer and don't use hand ratingarrow_forwardConvert the following structures into a chair representation. Then conduct a chair flip. Cl a. b. C\.... оarrow_forwardAktiv Learning App Cengage Digital Learning Part of Speech Table for Assign x o Mail-Karen Ento-Outlook * + app.aktiv.com Your Aktiv Learning trial expires on 02/06/25 at 01:15 PM Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 17 of 30 Drawing Arrows heat 4 O M B D 5x H H Und Settings H Done :0: H Jararrow_forward
- Gramicidin A can adopt more than one structure; NMR spectroscopy has revealed an “end-to-end” dimer form, and x-ray crystallography has revealed an “anti-parallel double- helical” form. Briefly outline and describe an experimentalapproach/strategy to investigate WHICH configuration (“end-to-end dimer” vs “anti-paralleldouble helical”) gramicidin adopts in an actual lipid bilayer.arrow_forwardDon't used hand raitingarrow_forwardCHEM2323 Problem 2-24 Tt O e: ל Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds. If the bonds needed are not drawn out, you should redraw them. + BF3 (a) (b) HI + (c) OH -BF Problem 2-25 Use curved arrows and a proton (H+) to draw the protonated form of the following Lewis bases. Before starting, add all missing lone pairs. (a) (b) :0: (c) N 1 CHEM2323 PS CH02 Name:arrow_forward
- CHEM2323 Problem 2-26 Tt O PS CH02 Name: Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right. (Draw all lone pairs first) (a) NH2 NH2 + (b) Problem 2-27 Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with H-Cl and draw the resulting carbocation. (a) H2C=CH2 (b) (c) Problem 2-28 Identify the most electronegative element in each of the following molecules: (a) CH2FCI F Problem 2-29 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li F 0 0 Use the electronegativity table in Figure 2.3 to predict which bond in the following pairs is more polar and indicate the direction of bond polarity for each compound. (a) H3C-Cl or Cl-CI (b) H3C-H or H-CI (c) HO-CH3 or (CH3)3Si-CH3 (d) H3C-Li or Li-OHarrow_forwardDon't used hand raitingarrow_forwardDon't used hand raitingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





