
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 21.4, Problem AQ
Interpretation Introduction
Interpretation:
The other functional group is identified in Capsaicin other than phenol.
Concept introduction:
Functional group: They are certain substitutes in the organic molecules which are determine the characteristic reactions taking place in it.
Amide:
A carbon atom is double-bonded to an oxygen atom
If the carbonyl carbon is attached with nitrogen is called as amide.
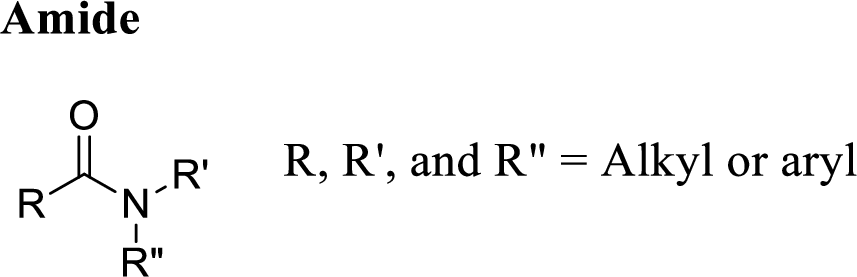
Alkenes are a class of hydrocarbons. The carbon-carbon double bond is called as alkenes and it is also called as olefins.
Ether:
The aliphatic or
Example is given below
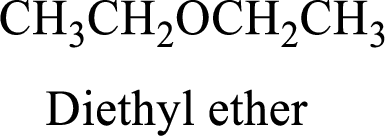
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Which of the following are descriptions of possible starting material for this
reaction?
H
?
trace acid
an ester
a ketone
an imine
an aldehyde
a carboxylic acid
an enamine
a primary amine
a secondary amine
a tertiary amine
None
What are the reagents needed for this and the third structure I only got the top right structure right
Chapter 21 Solutions
Organic Chemistry
Ch. 21.2 - Construct a Frost circle for a planar...Ch. 21.2 - Which compound gives a signal in the 1H-NMR...Ch. 21.2 - Prob. 21.3PCh. 21.2 - Prob. 21.4PCh. 21.3 - Prob. 21.5PCh. 21.4 - Arrange these compounds in order of increasing...Ch. 21.4 - Prob. AQCh. 21.4 - Prob. BQCh. 21.4 - Prob. CQCh. 21.5 - Prob. 21.7P
Ch. 21 - Name the following compounds and ions.Ch. 21 - Prob. 21.9PCh. 21 - Draw a structural formula for each compound. (a)...Ch. 21 - Molecules of 6,6-dinitrobiphenyl-2,2-dicarboxylic...Ch. 21 - Following each name is the number of Kekul...Ch. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Prob. 21.15PCh. 21 - Which of the molecules and ions given in Problem...Ch. 21 - Prob. 21.17PCh. 21 - Naphthalene and azulene are constitutional isomers...Ch. 21 - Prob. 21.19PCh. 21 - Prob. 21.20PCh. 21 - Following are IR and 1H-NMR spectra of compound D....Ch. 21 - Compound E (C8H10O2) is a neutral solid. Its mass...Ch. 21 - Following are 1H-NMR and 13C-NMR spectral data for...Ch. 21 - Following are 1H-NMR and 13C-NMR spectral data for...Ch. 21 - Compound H (C8H6O3) gives a precipitate when...Ch. 21 - Compound I (C11H14O2) is insoluble in water,...Ch. 21 - Propose a structural formula for compound J...Ch. 21 - Propose a structural formula for the analgesic...Ch. 21 - Prob. 21.29PCh. 21 - Prob. 21.30PCh. 21 - Given here are 1H-NMR and 13C-NMR spectral data...Ch. 21 - Prob. 21.32PCh. 21 - Prob. 21.33PCh. 21 - Prob. 21.34PCh. 21 - Arrange the molecules and ions in each set in...Ch. 21 - Prob. 21.36PCh. 21 - Prob. 21.37PCh. 21 - From each pair, select the stronger base.Ch. 21 - Prob. 21.39PCh. 21 - Prob. 21.40PCh. 21 - Prob. 21.41PCh. 21 - Prob. 21.42PCh. 21 - Following is an equation for iodination of...Ch. 21 - Prob. 21.44PCh. 21 - Prob. 21.45PCh. 21 - Prob. 21.46PCh. 21 - When warmed in dilute sulfuric acid,...Ch. 21 - In the chemical synthesis of DNA and RNA, hydroxyl...Ch. 21 - Prob. 21.49PCh. 21 - Write the products of the following sequences of...Ch. 21 - Prob. 21.51PCh. 21 - Show how to convert 1-phenylpropane into the...Ch. 21 - Prob. 21.53PCh. 21 - Cromolyn sodium, developed in the 1960s, has been...Ch. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Prob. 21.58PCh. 21 - Prob. 21.59PCh. 21 - Prob. 21.60PCh. 21 - Prob. 21.61PCh. 21 - Prob. 21.62PCh. 21 - Prob. 21.63PCh. 21 - Following is a synthesis for toremifene, a...
Knowledge Booster
Similar questions
- Please label this COZY spectraarrow_forwardPlease label this HNMRarrow_forwardConsider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B. Inject A B mixture Area= 9 Area = 5 Area = 3 Area Inject . མི། Inject J2 What is the percentage of Compound B in the the mixture?arrow_forward
- Rank these according to stability. CH3 H3C CH3 1 CH3 H3C 1 most stable, 3 least stable O 1 most stable, 2 least stable 2 most stable, 1 least stable O2 most stable, 3 least stable O3 most stable, 2 least stable O3 most stable, 1 least stable CH3 2 CH3 CH3 H₂C CH3 3 CH3 CHarrow_forwardConsider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forward
- What is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forwardWe added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forwardRank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forward
- O-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forwardK 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forwardI I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning