
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 21.11P
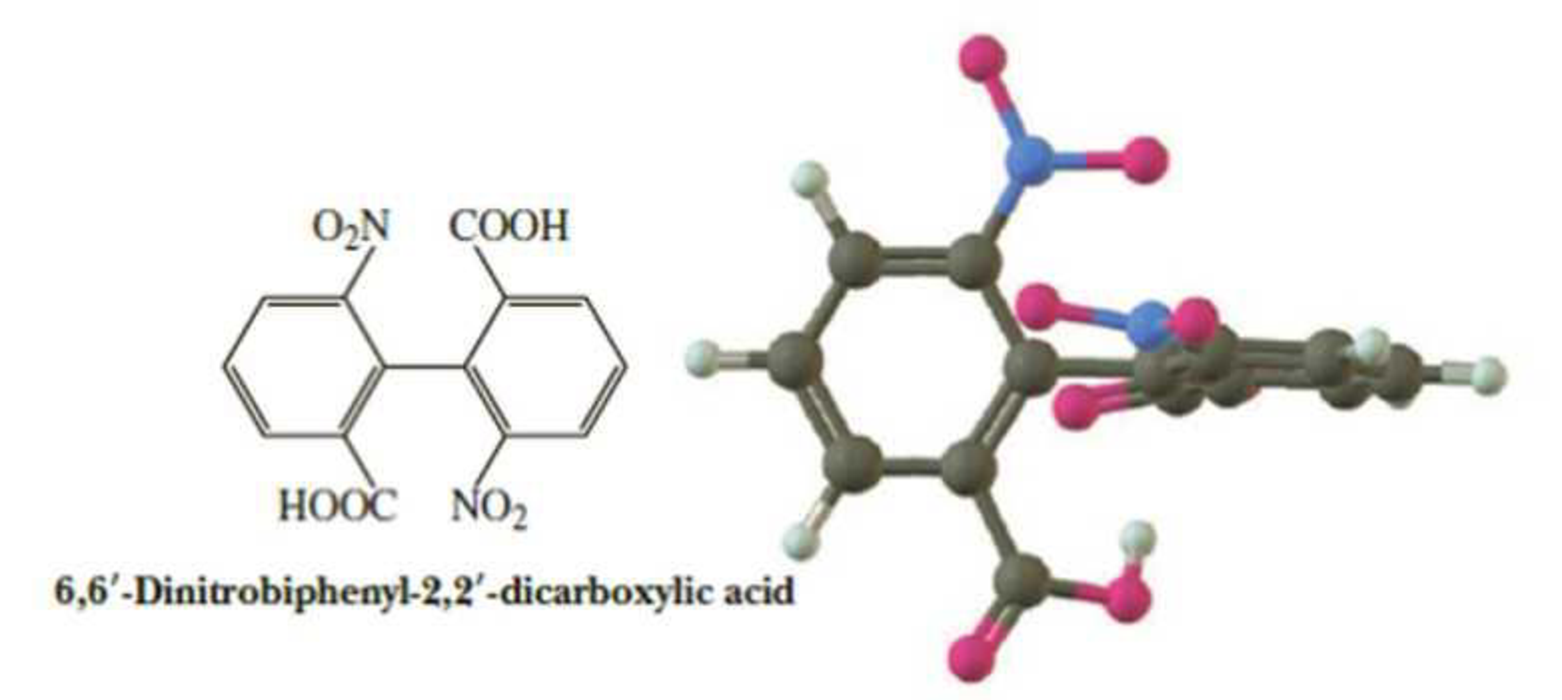
Molecules of 6,6′-dinitrobiphenyl-2,2′-dicarboxylic acid have no tetrahedral chiral center, and yet they can be resolved to a pair of enantiomers. Account for this chirality.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the formula of the product obtained by reacting adipic acid 1st with PCl5 and 2nd treatment with NH3.
please help me with my homework
help
Chapter 21 Solutions
Organic Chemistry
Ch. 21.2 - Construct a Frost circle for a planar...Ch. 21.2 - Which compound gives a signal in the 1H-NMR...Ch. 21.2 - Prob. 21.3PCh. 21.2 - Prob. 21.4PCh. 21.3 - Prob. 21.5PCh. 21.4 - Arrange these compounds in order of increasing...Ch. 21.4 - Prob. AQCh. 21.4 - Prob. BQCh. 21.4 - Prob. CQCh. 21.5 - Prob. 21.7P
Ch. 21 - Name the following compounds and ions.Ch. 21 - Prob. 21.9PCh. 21 - Draw a structural formula for each compound. (a)...Ch. 21 - Molecules of 6,6-dinitrobiphenyl-2,2-dicarboxylic...Ch. 21 - Following each name is the number of Kekul...Ch. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Prob. 21.15PCh. 21 - Which of the molecules and ions given in Problem...Ch. 21 - Prob. 21.17PCh. 21 - Naphthalene and azulene are constitutional isomers...Ch. 21 - Prob. 21.19PCh. 21 - Prob. 21.20PCh. 21 - Following are IR and 1H-NMR spectra of compound D....Ch. 21 - Compound E (C8H10O2) is a neutral solid. Its mass...Ch. 21 - Following are 1H-NMR and 13C-NMR spectral data for...Ch. 21 - Following are 1H-NMR and 13C-NMR spectral data for...Ch. 21 - Compound H (C8H6O3) gives a precipitate when...Ch. 21 - Compound I (C11H14O2) is insoluble in water,...Ch. 21 - Propose a structural formula for compound J...Ch. 21 - Propose a structural formula for the analgesic...Ch. 21 - Prob. 21.29PCh. 21 - Prob. 21.30PCh. 21 - Given here are 1H-NMR and 13C-NMR spectral data...Ch. 21 - Prob. 21.32PCh. 21 - Prob. 21.33PCh. 21 - Prob. 21.34PCh. 21 - Arrange the molecules and ions in each set in...Ch. 21 - Prob. 21.36PCh. 21 - Prob. 21.37PCh. 21 - From each pair, select the stronger base.Ch. 21 - Prob. 21.39PCh. 21 - Prob. 21.40PCh. 21 - Prob. 21.41PCh. 21 - Prob. 21.42PCh. 21 - Following is an equation for iodination of...Ch. 21 - Prob. 21.44PCh. 21 - Prob. 21.45PCh. 21 - Prob. 21.46PCh. 21 - When warmed in dilute sulfuric acid,...Ch. 21 - In the chemical synthesis of DNA and RNA, hydroxyl...Ch. 21 - Prob. 21.49PCh. 21 - Write the products of the following sequences of...Ch. 21 - Prob. 21.51PCh. 21 - Show how to convert 1-phenylpropane into the...Ch. 21 - Prob. 21.53PCh. 21 - Cromolyn sodium, developed in the 1960s, has been...Ch. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Prob. 21.58PCh. 21 - Prob. 21.59PCh. 21 - Prob. 21.60PCh. 21 - Prob. 21.61PCh. 21 - Prob. 21.62PCh. 21 - Prob. 21.63PCh. 21 - Following is a synthesis for toremifene, a...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
- 0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward9arrow_forward
- alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY