
Concept explainers
Distinguish precisely, and in scientific terms, the differences among items in each of the following pairs or groups.
a)
b) Saturated and
c)
d) Normal alkane, branched alkane
e) Cis and trans isomers
f) Structural formula, condensed (line) formula, molecular formula
g) Addition reaction, substitution reaction
h) Alkane, alkyl group
i) Monomer, polymer
j) Aliphatic
k) Ortho-, meta-, para
l) Alcohol,
m) Hydroxyl group, carbonyl group, carboxyl group
n) Primary, secondary, tertiary alcohols
o) Alcohol, ether
p) Aldehyde, ketone
q) Carboxylic acid, ester
r) Carboxylic acid, amide
s) Amine, amide
t) Primary, secondary, tertiary amine
u) Chain-growth polymer, step-growth polymer
(a)
Interpretation:
The difference between organic chemistry and inorganic chemistry is to be determined.
Concept introduction:
The compound is a substance that consists of the atoms connected to each other by chemical bonds. Compounds can be classified as organic and inorganic material on the basis of the material from which they are derived. The organic chemistry deals with the compounds that are obtained from plants, animals and living organisms and inorganic chemistry deals with the compounds that are obtained from minerals.
Answer to Problem 95E
The chemistry of minerals and the compounds derived from the minerals is known as inorganic chemistry.
The chemistry of the compounds that deals with the compounds that are derived from plants, animals and living organisms is known as organic chemistry. It can also be defined as the chemistry that deals with carbon compounds.
Explanation of Solution
Compounds can be classified as organic and inorganic material on the basis of the material from which they are derived.
The organic compound is the class of compound that has carbon-hydrogen bond or carbon-carbon long chains in their structure. They are derived from plants, animals and living organisms. For example, acetate ion
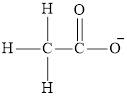
Inorganic compounds are those compounds that consist of other elements than carbon or have no carbon-carbon bond in their structure. They are derived from minerals. Cyanide ion
The chemistry of minerals and the compounds derived from the minerals is known as inorganic chemistry.
The chemistry of the compounds that deals with the compounds that are derived from plants, animals and living organisms is known as organic chemistry. It can also be defined as the chemistry that deals with carbon compounds.
(b)
Interpretation:
The difference between saturated and unsaturated hydrocarbons is to be determined.
Concept introduction:
Hydrocarbons are the binary compounds that consist of carbon and hydrogen atoms in its structure. Hydrocarbons can be classified into two types: saturated hydrocarbon and unsaturated hydrocarbon.
Saturated hydrocarbon consists of single bonds in its structure and unsaturated hydrocarbon consists of double or triple bond in its structure.
Answer to Problem 95E
Saturated hydrocarbons are those in which carbon atom is singly-bonded to other 4 other atoms. Unsaturated hydrocarbons consist of double or triple C-C bond in its structure.
Explanation of Solution
Hydrocarbons can be classified into two types: saturated hydrocarbon and unsaturated hydrocarbon.
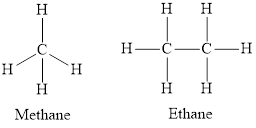
Saturated hydrocarbons are those in which each carbon atom is singly-bonded to four other atoms in the structure. Alkanes are an example of saturated hydrocarbons. In alkanes, each carbon atom is bonded four other atoms via single bond only. The general formula of alkanes is
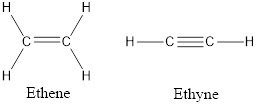
Unsaturated hydrocarbons consist of double or triple carbon-carbon bond in its structure and less than the maximum bond that carbon can make. Alkenes and alkynes are examples of an unsaturated hydrocarbon. Alkenes consist of a carbon-carbon double bond in its structure and alkynes consist of carbon-carbon triple bond in its structure. For example, ethene
Saturated hydrocarbons are those in which carbon atom is singly-bonded to other 4 other atoms. Unsaturated hydrocarbons consist of double or triple C-C bond in its structure.
(c)
Interpretation:
The difference between alkanes, alkenes, alkynes is to be determined.
Concept introduction:
Hydrocarbons are the binary compounds consist of carbon and hydrogen atoms in its structure. Alkanes, alkenes, and alkynes are examples of hydrocarbons.
Alkanes consist of a single bond, alkenes consist of double bond and alkynes consist of triple bond in its structure.
Answer to Problem 95E
Alkanes are the saturated hydrocarbons in which carbon atom is bonded to four other carbon atoms by a single bond. An alkene is a class of hydrocarbon that contains one C=C bond between two carbons. An alkyne is a class of hydrocarbon that contains a triple bond between C-C.
Explanation of Solution
Alkanes are the saturated hydrocarbons in which each carbon atom is bonded to four other atoms via single bond only. The general formula of alkanes is
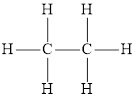
The structure of ethane is,
Alkenes are the unsaturated hydrocarbons that contain at least the one double bond between two carbons atoms. The general formula of alkenes is
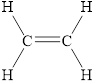
Alkynes are the unsaturated hydrocarbons that contain at least the one triple bond between two carbons atoms. The general formula of alkenes is
![]()
Alkanes are the saturated hydrocarbons in which each carbon atom is bonded to four other atoms via single bond only. An alkene is a class of hydrocarbon that contains at least the one double bond between two carbons. An alkyne is a class of hydrocarbon that contains at least the one triple bond between two carbons.
(d)
Interpretation:
The difference between normal alkane and branched alkane is to be determined.
Concept introduction:
Hydrocarbons are the binary compounds that consist of carbon and hydrogen atoms in its structure.
Alkanes are the saturated hydrocarbons in which each carbon atom is bonded to four other atoms via single bond only. The general formula of alkanes is
Normal alkanes are the alkanes that have a straight chain in their structure and branched alkanes are obtained by the substitution of alkyl group in place of a hydrogen atom in a normal alkane.
Answer to Problem 95E
If all the carbon atoms in alkane are arranged in a linear or continuous chain, the resulting hydrocarbon is called normal alkane. If all the carbon atoms in alkane are not arranged in a linear or continuous chain, the resulting hydrocarbon is called branched alkane.
Explanation of Solution
If all the carbon atoms in alkane are arranged in a linear or continuous chain, the resulting hydrocarbon is called normal alkane. Normal alkanes are also known as straight chain alkanes. The structural formula of a normal alkane is as follows,
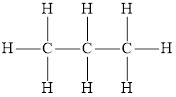
Branched alkanes are derived from the normal alkanes by substituting one or more hydrogen atom with an alkyl group.
The structural formula of a branched alkane is as follows,
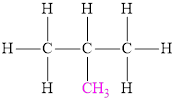
If all the carbon atoms in alkane are arranged in a linear or continuous chain, the resulting hydrocarbon is called normal alkane. If all the carbon atoms in alkane are not arranged in a linear or continuous chain, the resulting hydrocarbon is called branched alkane.
(e)
Interpretation:
The difference between cis and trans isomers is to be determined.
Concept introduction:
Isomers are the compounds which have the same molecular formula but a different arrangement of atoms. Alkenes have a double bond between carbon atoms. Due to the presence of a double bond, the rotation about adjacent carbon-carbon atoms is highly restricted. Hence, alkenes can exist in two forms: cis and trans isomers. In alkenes, when the two same groups are on the same side of the double bond then is known as cis-isomer and when the two same groups are on the opposite side of the double bond then is known trans-isomer.
Answer to Problem 95E
Cis isomer is the one in which the identical groups are placed on the same side of the double bond and trans isomer is the one in which the identical groups are placed on the different side of the double bond.
Explanation of Solution
An alkene is a molecule that contains at least the one double bond between two carbons atoms. Due to the presence of a double bond, the rotation about adjacent carbon-carbon atoms is highly restricted. Hence, alkenes can exist in two forms which have the same molecular formula but a different arrangement of atoms around the double bond. This isomerism is a type of stereoisomerism which is known as geometric isomerism or cis-trans isomerism, for example, 1, 2-dichloroethane can show the geometrical isomerism,
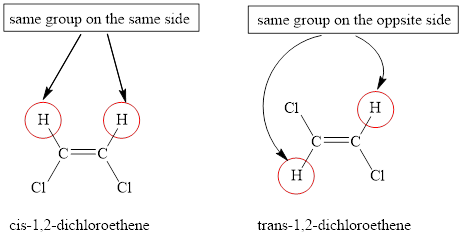
Cis isomer is the one in which the identical groups are placed on the same side of the double bond and trans isomer is the one in which the identical groups are placed on the different side of the double bond.
(f)
Interpretation:
The difference between structural formula, condensed (line) formula, the molecular formula is to be determined.
Concept introduction:
A hydrocarbon can be represented using different formulas. It can be represented using structural formula, structural diagram, line formula, condensed formula, or Lewis diagram.
Answer to Problem 95E
Structural formula represents the arrangement and bonding between the atoms without representing the unshared pairs of electrons. Line formula is the shorthand representation of the Lewis diagram, in which the bond between the parent chain carbon and atoms or other branches from the chain are not shown. Clubbing of
Explanation of Solution
The molecular formula is the simplest representation of the compound that tells about the number of atoms of each element present in the compound. Therefore, the molecular formula of an alkane having 7 carbon atoms is
In line formula, the bond between the chain carbon and atoms or other branches from the chain is not shown. It is the shorthand representation of the Lewis diagram of the molecule or compound. Therefore, the line formula of an alkane having 7 carbon atoms is
The condensed formula is the formula in which the
Structural formula represents the arrangement and bonding between the atoms without representing the unshared pairs of electrons. In alkanes, all the carbon atoms are bonded to four different atoms. Therefore, the structural formula is,
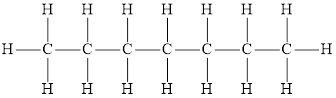
Structural formula represents the arrangement and bonding between the atoms without representing the unshared pairs of electrons. Line formula is the shorthand representation of the Lewis diagram, in which the bond between the parent chain carbon and atoms or other branches from the chain are not shown. Clubbing of
(g)
Interpretation:
The difference between the addition reaction and substitution reaction is to be determined.
Concept introduction:
Addition reaction involves the formation of a large molecule by the reaction of two molecules whereas substitution reaction involves the replacement of hydrogen atom by the other atom or group.
Answer to Problem 95E
In the addition reaction, two atoms are added on both sides of the multiple bonds.
In the substitution reaction, one or more hydrogen atoms from the reactant molecule are substituted by one or more other atoms or groups.
Explanation of Solution
An addition reaction is a reaction in which two molecules react to form one larger molecule. Unsaturated hydrocarbons (alkenes and alkynes) have carbon-carbon multiple bonds and thus undergo addition reaction. Alkenes and alkynes are capable of adding atoms of a molecule across the carbon-carbon double or triple bond respectively and give corresponding alkanes.
For example:
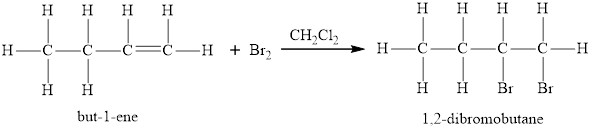
Alkanes give substitution reactions. In the substitution reaction, one or more hydrogen atoms from alkanes are substituted by one or more other atoms or groups in presence of heat and light. An example of a substitution reaction of alkanes is as follows:
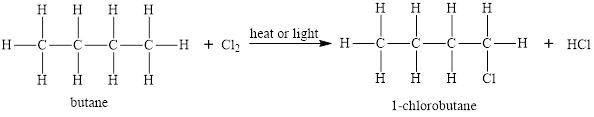
In the addition reaction, two atoms are added on both sides of the multiple bonds.
In the substitution reaction, one or more hydrogen atoms from the reactant molecule are substituted by one or more other atoms or groups.
(h)
Interpretation:
The difference between alkane and alkyl group is to be determined.
Concept introduction:
Hydrocarbons are the binary compounds that consist of carbon and hydrogen atoms in its structure.
Alkanes are the saturated hydrocarbons in which each carbon atom is bonded to four other atoms via single bond only. Alkyl group is derived from an alkane. It is an alkane with one hydrogen atom less than the corresponding alkane.
Answer to Problem 95E
An alkyl group is derived from the corresponding alkane. The general formula of an alkane is
Explanation of Solution
An alkyl group is derived from the corresponding alkane. Alkyl group is an alkane with one hydrogen atom less than the corresponding alkane. The alkyl group is named as the name of the alkane counterpart containing the same number of carbon atoms by replacing the suffix “ane” in the name with “yl”.
The molecular formula of an alkane with two carbon atoms is
The name of an alkane with two carbon atoms is ethane and the name of the alkyl group is ethyl.
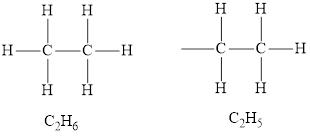
An alkyl group is derived from the corresponding alkane. The general formula of an alkane is
(i)
Interpretation:
The difference between monomer and polymer is to be determined.
Concept introduction:
Polymers are the macromolecules that are formed by the repeating monomeric units known as monomers.
Answer to Problem 95E
Monomers are small molecules that combine together in a sequential manner to produce a large molecule called polymer.
Explanation of Solution
Polymers are large compounds formed by polymerization of a number of repeated molecules called monomers. They are formed when the monomers are linked to one another by a suitable reaction like addition reaction or condensation reaction.

Monomers are small molecules that combine together in a sequential manner to produce a large molecule called polymer.
(j)
Interpretation:
The difference between aliphatic hydrocarbon and aromatic hydrocarbon is to be determined.
Concept introduction:
Hydrocarbons are the binary compounds that consist of carbon and hydrogen atoms in its structure. Aliphatic hydrocarbons are organic compounds that have open chain like structure and aromatic hydrocarbon have ring like structure with an alternate single and double bond in their structure.
Answer to Problem 95E
Aliphatic hydrocarbons are organic compounds in which carbon and hydrogen are bonded in the open chain or non-aromatic rings. Aliphatic hydrocarbons do not contain benzene ring in its structure. Aromatic hydrocarbons are cyclic compounds that contain the alternate single and double bond or benzene ring in its structure.
Explanation of Solution
Alkanes are an example of open chain aliphatic hydrocarbons and cycloalkanes are an example of closed or ring aliphatic hydrocarbons. Most of the alkanes exist in an open-chain for example:

Benzene is the simplest aromatic hydrocarbon and thus aromatic hydrocarbons are also defined as the compounds that contain benzene in its structure. The structure of benzene is,
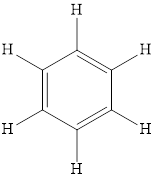
Aliphatic hydrocarbons are organic compounds in which carbon and hydrogen are bonded in the open chain or non-aromatic rings. Aliphatic hydrocarbons do not contain benzene ring in its structure. Aromatic hydrocarbons are cyclic compounds that contain the alternate single and double bond or benzene ring in its structure.
(k)
Interpretation:
The difference between ortho-, meta-, -para is to be determined.
Concept introduction:
Aromatic hydrocarbons exhibit aromaticity. Aromaticity is a property exhibited by conjugated cycloalkenes in which the stability is gained by the delocalization of the electrons in the pi-orbitals. Aromatic hydrocarbons contain one or more benzene rings. Benzene is the simplest aromatic hydrocarbon. Those hydrocarbons which do not contain a benzene ring are called aliphatic hydrocarbons.
Answer to Problem 95E
Ortho-, meta- and para- are prefixes used to denote the position of the substituent when two hydrogens of the benzene ring are substituted. If two hydrogens are substitute two adjacent hydrogen atoms the name of the compound is given by using the prefix ortho. If two hydrogens are substitute two hydrogen atoms attached to 1 and 3 carbon atom the name of the compound is given by using the prefix meta. If two hydrogens are substitute two hydrogen atoms attached to 1 and 4 carbon atom the name of the compound is given by using the prefix para.
Explanation of Solution
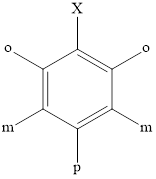
Benzene is the simplest aromatic hydrocarbon. The structure of benzene and the relative positions with respect to substituent X are as follows:
Xylene is the common name for dimethylbenzene. There are three isomers possible for xylene depending upon the position of the two methyl groups attached to the benzene ring are
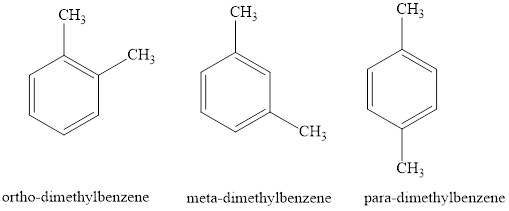
Ortho-, meta- and para- are prefixes used to denote the position of the substituent when two hydrogen of the benzene ring are substituted. If two hydrogens are substitute two adjacent hydrogen atoms the name of the compound is given by using the prefix ortho. If two hydrogen are substitute two hydrogen atoms attached to 1 and 3 carbon atom the name of the compound is given by using the prefix meta. If two hydrogens are substitute two hydrogen atoms attached to 1 and 4 carbon atom the name of the compound is given by using the prefix para.
(l)
Interpretation:
The difference between alcohol, aldehyde, and carboxylic acid is to be determined.
Concept introduction:
Concept introduction:
The properties of the compound are largely governed by the functional group present in the compound. The general representation of alcohol, aldehyde, and the carboxylic acid is
Answer to Problem 95E
The functional group determines the class of the compound to which it belongs. The properties of the compound are largely governed by the functional group present in the compound. Different functional groups have different characteristic properties.
| Functional groups | Structures |
Alcohol |
|
Aldehyde |
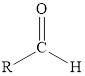 |
Carboxylic acid |
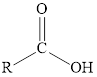 |
Explanation of Solution
Alcohol is a functional group which contains a hydroxyl group. The oxygen atom of the hydroxyl group is attached to the alkyl group via a single bond. Hydroxy group is obtained by removal of one hydrogen atom from the water molecule. Substitution of one hydrogen atom from the water by an alkyl group
![]()
An aldehyde is a functional group in which the carbonyl group is attached to the hydrogen atom on one side and alkyl group on another side.
A carboxyl group is a functional group containing a carbonyl group attached to a hydroxyl group via a single bond. A carboxyl group attached to an alkyl group forms a carboxylic acid.

The functional group determines the class of the compound to which it belongs. The properties of the compound are largely governed by the functional group present in the compound. Different functional groups have different characteristic properties.
| Functional groups | Structures |
Alcohol |
|
Aldehyde |
 |
Carboxylic acid |
 |
(m)
Interpretation:
The difference between the hydroxyl group, a carbonyl group, and a carboxyl group is to be determined.
Concept introduction:
The properties of the compound are largely governed by the functional group present in the compound. Hydroxyl group is present in alcohol, the carbonyl group in aldehyde or ketone and a carboxyl group in a carboxylic acid.
Answer to Problem 95E
The functional group determines the class of the compound to which it belongs. The properties of the compound are largely governed by the functional group present in the compound. Different functional groups have different characteristic properties.
| Functional groups | Identifies as | Structures |
Hydroxyl |
Alcohol |
|
Carbonyl |
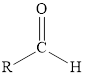
Aldehyde or ketone |
 |
Carboxyl |
Carboxylic acid |
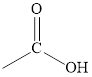 |
Explanation of Solution
A hydroxyl group is a functional group in which oxygen atom is bonded to a hydrogen atom via a single bond. Hydroxy group is obtained by removal of one hydrogen atom from the water molecule.
![]()
A carbonyl group is a functional group in which carbon is bonded to oxygen by a double bond.

A carboxyl group is a functional group containing a carbonyl group attached to a hydroxyl group via a single bond.
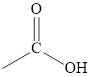
The functional group determines the class of the compound to which it belongs. The properties of the compound are largely governed by the functional group present in the compound. Different functional groups have different characteristic properties.
| Functional groups | Identifies as | Structures |
Hydroxyl |
Alcohol |
|
Carbonyl |
Aldehyde or ketone |
 |
Carboxyl |
Carboxylic acid |
 |
(n)
Interpretation:
The difference between primary, secondary, and tertiary alcohols is to be determined.
Concept Introduction:
Alcohols are organic compounds containing a
Answer to Problem 95E
(1) Primary alcohols: Alcohol in which the hydroxyl group is attached to the primary carbon atom is called primary alcohol.
(2) Secondary alcohols: Alcohol in which the hydroxyl group is attached to the secondary carbon atom is called secondary alcohol.
(3) Tertiary alcohols: Alcohol in which the hydroxyl group is attached to the tertiary carbon atom is called tertiary alcohol.
Explanation of Solution
Alcohol is a functional group which contains a hydroxyl group. The oxygen atom of the hydroxyl group is attached to the alkyl group via a single bond. Hydroxy group is obtained by removal of one hydrogen atom from the water molecule. Substitution of one hydrogen atom from the water by an alkyl group
![]()
A tertiary carbon
A secondary carbon
A primary carbon
The structural formula of primary alcohol, secondary and tertiary alcohol is,
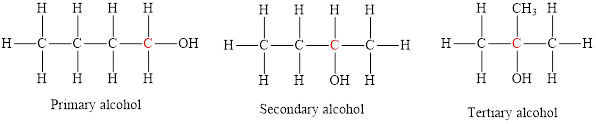
Primary alcohols: Alcohol in which the hydroxyl group is attached to the primary carbon atom is called primary alcohol. Secondary alcohols: Alcohol in which the hydroxyl group is attached to the secondary carbon atom is called secondary alcohol. Tertiary alcohols: Alcohol in which the hydroxyl group is attached to the tertiary carbon atom is called tertiary alcohol.
(o)
Interpretation:
The difference between alcohol and ether is to be determined.
Concept Introduction:
Alcohols are organic compounds containing a

Ethers are organic compounds containing an oxygen atom intervened between two alkyl groups. Ethers are derived from alkanes by replacing a hydrogen atom for an alkoxy group. The structure of ethers is not held together by hydrogen bonds. Due to very low polarity, ethers are not very well soluble in water.
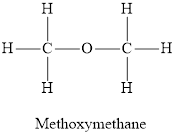
Answer to Problem 95E
Alcohols and ethers both have the same general formula
Explanation of Solution
Alcohol is an organic compound in which a hydroxyl group is attached to the carbon chain. The general representation of alcohol is
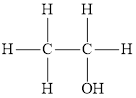
Ether is an organic compound in which oxygen atom is attached with two alkyl groups. The general representation of ether is
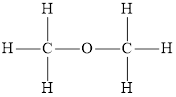
Alcohols and ethers both have the same general formula
(p)
Interpretation:
The difference between aldehyde and ketone is to be determined.
Concept Introduction:
Aldehydes are organic compounds containing a
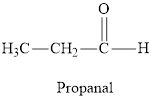
Ketones are organic compounds in which two alkyl/aryl groups are intervened by a
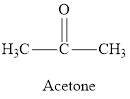
Answer to Problem 95E
Aldehyde and ketone both have carbonyl group but in ketone, the carbonyl group is attached to the alkyl group on either side whereas in aldehyde it is attached to the hydrogen atom on one side and to alkyl group on another side.
Explanation of Solution
Aldehyde and ketones are characterized by the carbonyl group. If the carbonyl group is attached to the hydrogen atom on one side and to an alkyl group on the other side the compound formed is called aldehyde and if an alkyl group is attached to either side of the carbonyl group the compound formed is called ketone.
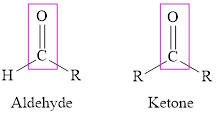
Aldehyde and ketone both have carbonyl group but in ketone, the carbonyl group is attached to the alkyl group on either side whereas in aldehyde it is attached to the hydrogen atom on one side and to alkyl group on another side.
(q)
Interpretation:
The difference between carboxylic acid and ester is to be determined.
Concept Introduction:
Esters are derivatives of carboxylic acids. Esters are more polar than ethers but less polar than alcohols. There is no intra-molecular hydrogen bonding in esters and hence their boiling points are significantly lower than those of acid with the same number of carbon atoms. Esters have pleasant odors and give a characteristic smell to fruits and flowers.
Carboxylic acids are compounds characterized by the presence of a
Answer to Problem 95E
The carboxylic acid is an organic acid that contains carbonyl group attached to a hydroxyl group whereas in ester the carbonyl group is attached to an alkyl group.
Explanation of Solution
The carboxylic acid consists of the carboxyl group in its structure that is bonded to the alkyl group. The general representation of carboxylic acid is,
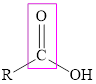
Ester also consists of a carboxyl group in its structure. In ester, the carboxyl carbon can be bonded to hydrogen or alkyl group but the oxygen of the carboxyl group must be bonded to an alkyl group. The general representation of ester is,
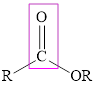
The carboxylic acid is an organic acid that contains carbonyl group attached to a hydroxyl group whereas in ester the carbonyl group is attached to an alkyl group.
(r)
Interpretation:
The difference between carboxylic acid and amide is to be determined.
Concept Introduction:
Carboxylic acids are compounds characterized by the presence of a

Amides are organic compounds represented as
Answer to Problem 95E
The carboxylic acid is an organic acid that contains carbonyl group attached to a hydroxyl group whereas in amide the carbonyl group is attached to an amine group.
Explanation of Solution
The carboxylic acid consists of the carboxyl group in its structure that is bonded to the alkyl group. The general representation of carboxylic acid is,
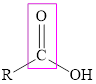
Amides are the derivatives of carboxylic acids in which the hydroxyl group is replaced by an amine. The structure of the corresponding amide group is,
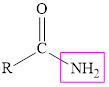
The carboxylic acid is an organic acid that contains carbonyl group attached to a hydroxyl group whereas in amide the carbonyl group is attached to an amine group.
(s)
Interpretation:
The difference between amine and amide is to be determined.
Concept Introduction:
Amines are compounds which contain a base nitrogen atom with a lone pair. The lower aliphatic amines are gaseous in nature. Lower aliphatic amines can form hydrogen bonds with water molecules. Hence such amines are water soluble. As the number of alkyl groups increases in the amines, their hydrophobic nature increases as well and hence, the solubility in water decreases. Amides are organic compounds represented as
Answer to Problem 95E
In amines, the nitrogen atom is attached to the one, two, or three alkyl group whereas in amides the nitrogen atom is attached to a carbonyl group. Also, amines are derivative of ammonia and amides are derivative of carboxylic acid.
Explanation of Solution
The nitrogen-containing organic compounds are known as amines. They are derivatives of ammonia. Substitution of one, two or three hydrogen atoms in the ammonia molecule by an alkyl group form an amine. Amines can be classified as primary, secondary or tertiary depending upon how many alkyl groups are attached to the nitrogen atom in amines.
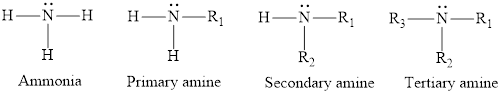
Amides are the derivatives of carboxylic acids in which the hydroxyl group is replaced by an amine. The structure of the corresponding amide group is,

In amines, the nitrogen atom is attached to the one, two, or three alkyl group whereas in amides the nitrogen atom is attached to a carbonyl group. Also, amines are derivative of ammonia and amides are derivative of carboxylic acid.
(t)
Interpretation:
The difference between primary, secondary, and tertiary amine is to be determined.
Concept Introduction:
Amines are compounds which contain a base nitrogen atom with a lone pair. The lower aliphatic amines are gaseous in nature. Lower aliphatic amines can form hydrogen bonds with water molecules. Hence such amines are water soluble. As the number of alkyl groups increases in the amines, their hydrophobic nature increases as well and hence, the solubility in water decreases. Primary and secondary amines are often engaged in the intermolecular association as a result of hydrogen bonding between the nitrogen of one and hydrogen of another molecule. In tertiary amines, there is no such association. Hence the order of boiling points of amines follows the order, Primary > Secondary > Tertiary.
Answer to Problem 95E
On the basis of the number of alkyl groups, three types of amines are classified as,
1. Primary amines: Amines in which the nitrogen atom is attached to one alkyl group and two hydrogen atoms are called primary amine.
2. Secondary amines: Amines in which the nitrogen atom is attached to two alkyl groups and one hydrogen atom are called secondary amine.
3. Tertiary amines: Amines in which the nitrogen atom is attached to three alkyl groups and zero hydrogen atom are called tertiary amine.
Explanation of Solution
The nitrogen-containing organic compounds are known as amines. They are derivatives of ammonia. Substitution of one, two or three hydrogen atoms in the ammonia molecule by an alkyl group form an amine. Amines can be classified as primary, secondary or tertiary depending upon how many alkyl groups are attached to the nitrogen atom in amines.
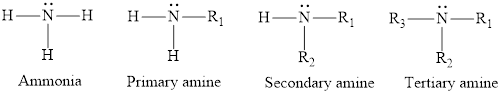
Amines are called, the organic derivatives of ammonia. In an ammonia molecule, one nitrogen atom is bonded to three hydrogen atoms. When one, two or three hydrogen atoms are replaced by either an alkyl or an aryl group amine is formed. Replacement of one hydrogen atom by an alkyl group gives primary amine. Replacement of two hydrogen atoms gives secondary amines, whereas all the three hydrogen atoms when replaced by alkyl/aryl groups, tertiary amines are formed.
(u)
Interpretation:
The difference between the chain-growth polymer and the step-growth polymer is to be determined.
Concept Introduction:
Polymerization is a process in which two or more monomer units combine to form long chains or three-dimensional networks.
Based on the modes of polymerization, polymers are classified as follows:
Addition polymers – Addition polymers are formed by repeated addition of the monomer units. The monomer units are unsaturated hydrocarbons generally. There is no elimination of molecules in the reaction.
Condensation polymers – Condensation polymers are formed by repeated condensation between two different monomer units along with the formation of elimination products like water.
Answer to Problem 95E
Chain growth polymers are those polymers that are synthesized from the addition reaction and step-growth polymers are those polymers that are synthesized from the condensation reaction.
Explanation of Solution
Chain growth polymers are the molecules that are synthesized from the addition reaction. In chain growth polymerization, one monomer adds to the growing polymer chain in a sequential manner resulting in a large chain-growth polymer. Generally, alkenes act as the monomer unit in these polymers.
The polymerization initiates when one bond between the carbon-carbon double bond breaks. This leads to the formation of new monomer units in which there is one unpaired electron on both atoms of the carbon double bond. The unpaired electron on the carbon atom of one monomer unit forms a bond with the neighboring monomer unit to form the polymer. The general three repeating units of the chain-growth polymer is,
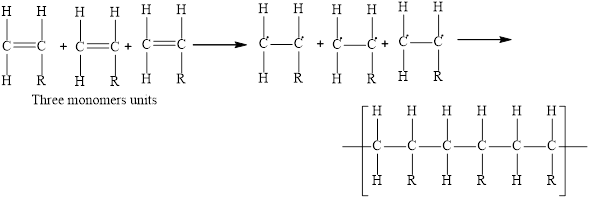
Here, R is an alkyl group.
Step-growth polymers are the polymers that are synthesized by the stepwise reaction of monomer unit with the growing chain. Condensation reactions are employed for the synthesis of the step-growth polymerization reaction.
The condensation reaction of the dicarboxylic acid and diamine are employed for the synthesis of polyesters. In the condensation reaction, one molecule of water is released resulting in the formation of an amide bond
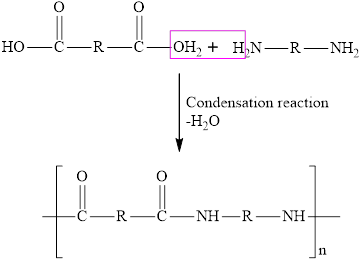
Chain growth polymers are those polymers that are synthesized from the addition reaction and step-growth polymers are those polymers that are synthesized from the condensation reaction.
Want to see more full solutions like this?
Chapter 21 Solutions
Introductory Chemistry: An Active Learning Approach
- Comment on the following paragraph. In halides, MXn stoichiometry does not require a value of n so large as to prevent the approach of M+ ions, for steric or electrostatic reasons.arrow_forwardExplain Wade's rules, Indicate what the letters S and n represent in the formula.arrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- Hi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forwardHi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





