
Concept explainers
(a)
Interpretation:
Whether the statement “to be classified as organic, a compound must be or have been a part of a living organism” is true or false is to be determined.
Concept introduction:
Compounds can be classified as organic and inorganic material on the basis of the material from which they are derived. The organic compound is the class of compound that has carbon-hydrogen bond or carbon-carbon long chains in their structure. They are derived from plants, animals and living organisms.
Inorganic compounds are those compounds that consist of other elements than carbon or have no carbon-carbon bond in their structure. They are derived from minerals.
Answer to Problem 96E
The statement “to be classified as organic, a compound must be or have been a part of a living organism”, is false.
Explanation of Solution
According to the old definition, all organic compounds are derived from living organisms. But the modern definition of an organic compound classifies organic compounds as the compound that has carbon-hydrogen bond or carbon-carbon long chains in their structure. They may either be derived from living plants or animals or may be produced by artificial means. The derivation of an organic compound from a living source is not mandatory anymore.
The statement “to be classified as organic, a compound must be or have been a part of a living organism”, is false.
(b)
Interpretation:
Whether the statement “carbon atoms normally form four bonds in organic compounds” is true or false is to be determined.
Concept introduction:
Carbon atom is tetravalent in nature. The simplest organic compound is methane. In a methane molecule, a carbon atom is covalently bonded to four hydrogen atoms. This is the maximum valency of a carbon atom can show.
Answer to Problem 96E
The statement “carbon atoms normally form four bonds in organic compounds” is true.
Explanation of Solution
The carbon atom has a valency of four. The four valence electrons in a carbon atom are present in the 2s and 2p orbitals. Hence carbon atom covalently binds to four atoms with four sigma bonds.
The statement “carbon atoms normally form four bonds in organic compounds” is true.
(c)
Interpretation:
Whether the statement “only an
Concept Introduction:
An addition reaction is defined as a reaction in which two or more molecules combine to form a larger one. An addition reaction occurs when atoms or groups are added to a compound containing multiple bonds. An example of an addition reaction is given below:

The atoms H and X in the HX molecule add to the carbon atoms of the ethene molecule to give halo-ethane molecule as the product.
Answer to Problem 96E
The statement “only an unsaturated hydrocarbon can engage in an addition reaction” is true.
Explanation of Solution
In a saturated hydrocarbon, all the four valences of all the carbon atoms are satisfied and hence these carbon atoms of saturated compounds are not reactive towards addition reactions. However, unsaturated hydrocarbons unlike their saturated counterparts, engage in an addition reaction. The multiple bonds present in the unsaturated hydrocarbons enable the compound to undergo addition reaction and add the molecule across the multiple bonds.
For example,
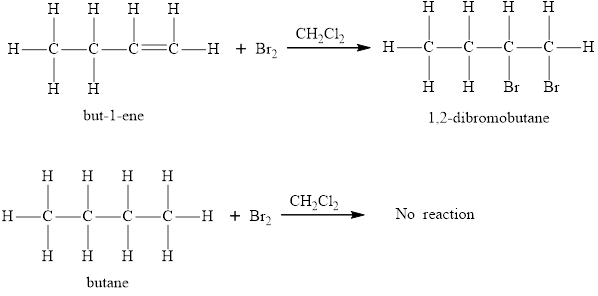
The statement “only an unsaturated hydrocarbon can engage in an addition reaction” is true.
(d)
Interpretation:
Whether the statement “members of a homologous series differ by a distinct structural unit” is true or false is to be determined.
Concept Introduction:
A homologous series is a series of compounds containing the same
Answer to Problem 96E
The statement, “members of a homologous series differ by a distinct structural unit” is true.
Explanation of Solution
For example, in
The statement, “members of a homologous series differ by a distinct structural unit” is true.
(e)
Interpretation:
Whether the statement “alkanes,
Concept Introduction:
Alkanes are hydrocarbons in which each carbon atoms is bonded with four other atoms through four single bonds. The general formula of alkanes is
Alkenes are those hydrocarbons which contain at least one carbon-carbon double bond. They are also called olefins. Alkenes are represented by the formula
Alkynes are hydrocarbons with one or more triple bonds between the carbon atoms. They are generally more reactive than alkanes and alkenes. They are represented by the general formula. Alkynes are represented by the general formula
Answer to Problem 96E
The statement “alkanes, alkenes and alkynes are unsaturated hydrocarbons” is false.
Explanation of Solution
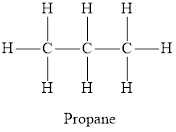
The chemical structure of an alkane is below
The chemical structure of an alkene and an alkyne are depicted below respectively.
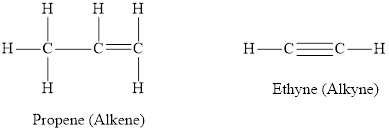
Hydrocarbon in which each carbon atom is bonded to 4 other atoms and does not contain any double or triple bond are called saturated hydrocarbons. Hydrocarbons in which at least one carbon-carbon triple or a double bond is present are called unsaturated hydrocarbons. Each carbon atom in propane is bonded via a single bond to 4 other atoms. Thus it is a saturated compound. In propene and ethyne not all carbon atoms are bonded to 4 other atoms by single bond. Propene has one double bond and ethyne has one triple bond thus propene and ethyne are unsaturated hydrocarbons.
The statement “alkanes, alkenes and alkynes are unsaturated hydrocarbons” is false.
(f)
Interpretation:
Whether the statement “alkyl groups are a class of organic compounds” is true or false is to be determined.
Concept Introduction:
An alkane is represented by the formula
Answer to Problem 96E
The statement “alkyl groups are a class of organic compounds” is false.
Explanation of Solution
An alkyl group is an alkane which has a deficiency of a single hydrogen atom. It has the general formula
The statement “alkyl groups are a class of organic compounds” is false.
(g)
Interpretation:
Whether the statement “isomers have the same molecular formulas but different structural formulas” is true or false is to be determined.
Concept Introduction:
Isomers are two or more molecules which have the same molecular formula but differ structurally. The two main forms of isomerism are structural isomerism and stereoisomerism.
When the atoms and functional are arranged in different ways, structural isomerism is exhibited. The types of structural isomerism are, chain, position, functional group metamerism and tautomerism.
Stereoisomers are those in which the spatial arrangement of atoms and functional groups is different. The two types of stereoisomers are geometrical and optical. In geometrical isomerism, the isomers possess the same structural formula but the spatial arrangement of the groups around the double bond. This isomerism is exhibited by alkenes and its derivatives. Optical isomerism is generally exhibited by molecules containing asymmetric carbon atoms. This results in two isomers which are mirror images of one another.
Answer to Problem 96E
The statement “isomers have the same molecular formulas but different structural formulas” is true.
Explanation of Solution
The structures of two isomers, pentane and
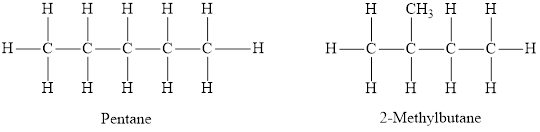
Both pentane and
The statement “isomers have the same molecular formulas but different structural formulas” is true.
(h)
Interpretation:
Whether the statement “cis-trans isomerism appears among alkenes but not alkynes” is true or false is to be determined.
Concept Introduction:
Stereoisomers are those in which the spatial arrangement of atoms and functional groups is different. The two types of stereoisomers are geometrical and optical. In geometrical isomerism, the isomers possess the same structural formula but the spatial arrangement of the groups around the double bond. This isomerism is exhibited by alkenes and its derivatives.
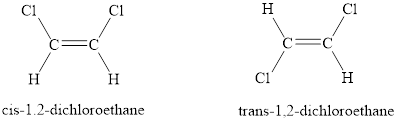
When similar groups lie on the same side, it is referred to as cis isomerism. When similar groups lie on the opposite sides, it is referred to as trans isomerism.
Answer to Problem 96E
The statement “cis-trans isomerism appears among alkenes buy not alkynes” is true.
Explanation of Solution
When the two same groups are on the same side of the double bond then is known as cis-isomer and when the two same groups are on the opposite side of the double bond then is known trans-isomer.
The condition for cis-trans isomerism is that the double bonded carbon atoms must have two different substituents attached at each end.
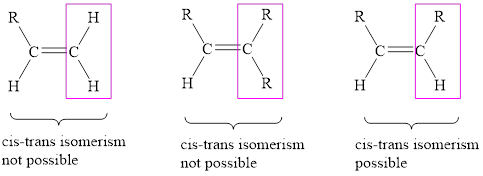
In alkenes, carbon atoms are bonded to three other atoms/groups due to their
In alkynes, each carbon atom can bond with two other atoms only. To show cis-trans isomerism there must present two groups on each carbon atom. Therefore, alkynes cannot show cis-trans isomerism. Like in the case of acetylene below
![]()
The statement “cis-trans isomerism appears among alkenes buy not alkynes” is true.
(i)
Interpretation:
Whether the statement “all aliphatic hydrocarbons are unsaturated” is true or false is to be determined.
Concept Introduction:
Aliphatic compounds are those hydrocarbons which contain carbon and hydrogen joined together in straight chains, branched chains or non-aromatic rings. The simplest aliphatic compound is the molecule of methane
The structures of aliphatic hydrocarbons are below:
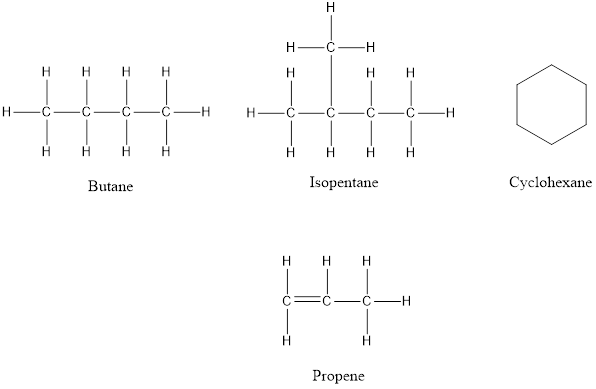
Answer to Problem 96E
The statement “all aliphatic hydrocarbons are unsaturated” is false.
Explanation of Solution
Aliphatic compounds are the hydrocarbons in which carbon and hydrogen are linked in a single chain, branches or non-aromatic rings. Aliphatic hydrocarbons include both saturated and unsaturated hydrocarbons. Hence the statement “all aliphatic hydrocarbons are unsaturated” is false.
The statement “all aliphatic hydrocarbons are unsaturated” is false.
(j)
Interpretation:
Whether the statement “aromatic hydrocarbons have a ringed structure” is true or false is to be determined.
Concept Introduction:
Aromatic hydrocarbons exhibit aromaticity. Aromaticity is a property exhibited by conjugated cycloalkenes in which the stability is gained by the delocalisation of the electrons in the pi-orbitals. Aromatic hydrocarbons contain one or more benzene rings. Benzene is the simplest aromatic hydrocarbon. Those hydrocarbons which do not contain a benzene ring are called aliphatic hydrocarbons.
Answer to Problem 96E
The statement “aromatic hydrocarbons have a ringed structure” is true.
Explanation of Solution
For a compound to be aromatic, there are three pre-requisites. Those are as follows:
The compound must be cyclic
The compound must be planar
The compound must follow
The foremost condition for a compound to exhibit aromaticity requires it to have a ringed structure. Hence the statement “aromatic hydrocarbons have a ringed structure” is true.
The statement “aromatic hydrocarbons have a ringed structure” is true
(k)
Interpretation:
Whether the statement “an alcohol has one alkyl group bonded to an oxygen atom, and ether has two” is true or false is to be determined.
Concept Introduction:
Alcohols are organic compounds containing a
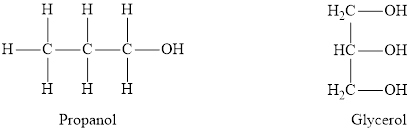
Ethers are organic compounds containing an oxygen atom intervened between two alkyl groups. Ethers are derived from alkanes by replacing a hydrogen atom for an alkoxy group. The structure of ethers is not held together by hydrogen bonds. Due to very low polarity, ethers are not very well soluble in water.
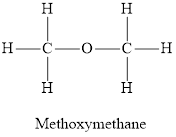
Answer to Problem 96E
The statement “an alcohol has one alkyl group bonded to an oxygen atom, and ether has two” is true.
Explanation of Solution
A water molecule has a structure as follows
![]()
Removal of a hydrogen atom gives us the hydroxyl group. This functional group is classified as alcohol. When one of the hydrogen atoms is replaced by an alkyl group an alcohol is formed.
![]()
Replacement of both the hydrogen atoms by an alkyl group each produces an ether molecule. Hence in this condition, the oxygen atom is bonded to two alkyl groups.
![]()
The statement “an alcohol has one alkyl group bonded to an oxygen atom, and ether has two” is true.
(l)
Interpretation:
Whether the statement “carbonyl groups are found in alcohols and aldehydes” is true or false is to be determined.
Concept Introduction:
Carbonyl compounds are molecules containing the carbonyl group. Compounds containing carbonyl groups are called aldehydes and ketones. In aldehydes, the carbonyl group is on the end of a carbon chain, while in ketones, it is in the middle of the carbon chain. The names of aldehydes and ketones are simply derived by dropping "-e" from the root and adding "-al" or "-one" respectively.
Answer to Problem 96E
The statement “carbonyl groups are found in alcohols and aldehydes” is false.
Explanation of Solution
A carbonyl group is a functional group in which a carbon atom is doubly bonded to an oxygen atom. In aldehydes, the oxygen atom is doubly bonded to a carbon atom. Hence aldehydes are carbonyl compounds. In alcohols, the oxygen atom is bonded to a carbon atom through a single bond and also with a hydrogen atom with a single bond. Hence alcohols cannot be classified as carbonyl compounds.
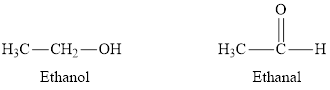
The statement “carbonyl groups are found in alcohols and aldehydes” is false.
(m)
Interpretation:
Whether the statement “an ester is an aromatic hydrocarbon, made by the reaction of an alcohol with a carboxylic acid” is true or false is to be determined.
Concept Introduction:
Esters are derivatives of carboxylic acids. Esters are more polar than ethers but less polar than alcohols. There is no intra-molecular hydrogen bonding in esters and hence their boiling points are significantly lower than those of acid with the same number of carbon atoms. Esters have pleasant odours and give characteristic smell to fruits and flowers.
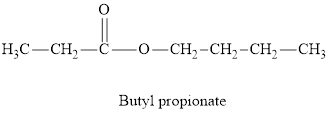
Answer to Problem 96E
The statement “an ester is an aromatic hydrocarbon made by the reaction of an alcohol with a carboxylic acid” is false.
Explanation of Solution
An ester is an organic compound with a general formula
Esters can be both aliphatic and aromatic. Hence the statement “an ester is an aromatic hydrocarbon made by the reaction of an alcohol with a carboxylic acid” is false”.
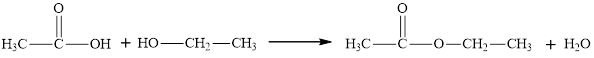
The statement “an ester is an aromatic hydrocarbon made by the reaction of an alcohol with a carboxylic acid” is false.
(n)
Interpretation:
Whether the statement “an amine has one, two, or three alkyl groups substituted for hydrogens in an ammonia molecule” is true or false is to be determined.
Concept Introduction:
Amines are compounds which contain a base nitrogen atom with a lone pair. The lower aliphatic amines are gaseous in nature. Lower aliphatic amines can form hydrogen bonds with water molecules. Hence such amines are water soluble. As the number of alkyl groups increases in the amines, their hydrophobic nature increases as well and hence, the solubility in water decreases. Primary and secondary amines are often engaged in the intermolecular association as a result of hydrogen bonding between the nitrogen of one and hydrogen of another molecule. In tertiary amines, there is no such association. Hence the order of boiling points of amines follows the order, Primary > Secondary > Tertiary.
Answer to Problem 96E
The statement “an amine has one, two or three alkyl groups substituted for hydrogens in an ammonia molecule” is true.
Explanation of Solution
Amines are called, the organic derivatives of ammonia. In an ammonia molecule, one nitrogen atom is bonded to three hydrogen atoms. When one, two or three hydrogen atoms are replaced by either an alkyl or an aryl group amine is formed. Replacement of one hydrogen atom by an alkyl group gives primary amine. Replacement of two hydrogen atoms gives secondary amines, whereas all the three hydrogen atoms when replaced by alkyl/aryl groups, tertiary amines are formed.
The statement “an amine has one, two or three alkyl groups substituted for hydrogens in an ammonia molecule” is true.
(o)
Interpretation:
Whether the statement “an amide has the structure of a carboxylic acid, except that
Concept Introduction:
Amides are organic compounds represented as
Answer to Problem 96E
The statement “an amide has the structure of a carboxylic acid, except that
Explanation of Solution
Amides are the derivatives of carboxylic acids in which the hydroxyl group is replaced by an amine.
The structures of an amide and a carboxylic acid are depicted below.

It is clear from the structures that in the amide, the
The statement “an amide has the structure of a carboxylic acid, except that
(p)
Interpretation:
Whether the statement “a peptide linkage arises when a water molecule forms from a hydrogen from the
Concept Introduction:
Peptide bond is formed when the carbonyl group of one amino acid becomes linked to the amino group of another to form a peptide. Amino acids in protein are joined together by peptide bonds.
Answer to Problem 96E
The statement “a peptide linkage arises when a water molecule forms from a hydrogen from the
Explanation of Solution
The formation of a peptide bond is a condensation reaction in which a water molecule is eliminated. Amino acids combine together to form polypeptides. A peptide bond is formed between two molecules of amino acids when the carboxyl group of one molecule reacts with the amino group of the other amino acid molecule. A molecule of water is released in the process.
A diagrammatic representation of peptide linkage formation is as follows,
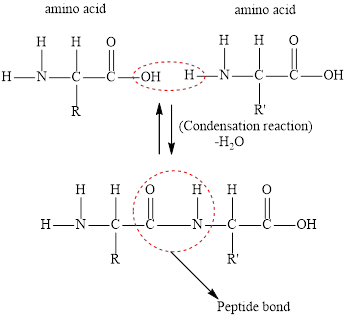
The statement “a peptide linkage arises when a water molecule forms from a hydrogen from the
(q)
Interpretation:
Whether the statement “forming cross-links in a polymer makes the polymer more likely to possess low mechanical strength” is true or false is to be determined.
Concept Introduction:
Based on the structures, polymers are classified as follows:
Linear polymers – In this category of polymers, the monomer units join each other to form long straight chains.
Branched polymers – When linear chain polymers undergo branching, they form branched chain polymers. They have low density and melting points. The melting point decreases upon increase in branching.
Cross linked polymers – These polymers are formed by formation of covalent bonds between multiple linear polymer chains.
Answer to Problem 96E
The statement “forming cross-links in a polymer makes the polymer more likely to possess low mechanical strength” is false.
Explanation of Solution
A cross-link is a bond in which one polymer chain is linked to another chain. These links may either be covalent or ionic in nature. Formation of cross-links increases the molar mass of the polymer chain and hence the mechanical strength of the polymer increases.
The statement “forming cross-links in a polymer makes the polymer more likely to possess low mechanical strength” is false.
(r)
Interpretation:
Whether the statement “chain-growth polymers are made by a reaction that gives off water as a second product” is true or false is to be determined.
Concept Introduction:
Based on the modes of polymerization, polymers are classified as follows:
Addition polymers – Addition polymers are formed by repeated addition of the monomer units. The monomer units are unsaturated hydrocarbons generally. There is no elimination of molecules in the reaction.
Condensation polymers – Condensation polymers are formed by repeated condensation between two different monomer units along with the formation of elimination products like water.
Answer to Problem 96E
The statement “chain-growth polymers are made by a reaction that gives off water as a second product” is false.
Explanation of Solution
The polymerization process by which water is released as a second product is called condensation or step growth polymerization. Chain growth polymers are made by addition or chain growth reactions. The monomer units in addition polymerization are unsaturated. The polymerization begins when one bond of the double bond between carbon atoms is broken in a molecule. This generates a new monomer with a broken bond. Each successive carbon atom is hence left with an unshared electron with which it forms a bond with a neighbouring molecule.
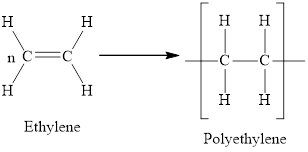
The statement “chain-growth polymers are made by a reaction that gives off water as a second product” is false.
(s)
Interpretation:
Whether the statement “nylon is an example of a step-growth condensation polymerization” is true or false is to be determined.
Concept Introduction:
Polymerization is a process in which two or more monomer units combine to form long chains or three dimensional networks.
Based on the modes of polymerization, polymers are classified as follows:
Addition polymers – Addition polymers are formed by repeated addition of the monomer units. The monomer units are unsaturated hydrocarbons generally. There is no elimination of molecules in the reaction.
Condensation polymers – Condensation polymers are formed by repeated condensation between two different monomer units along with the formation of elimination products like water.
Answer to Problem 96E
The statement “nylon is an example of a step-growth condensation polymerization” is true.
Explanation of Solution
Nylon 6-6 is made by the condensation polymerization of hexa-methylenediamine and adipic acid. A water molecule is eliminated after it is formed by the combination of a
The reaction is,
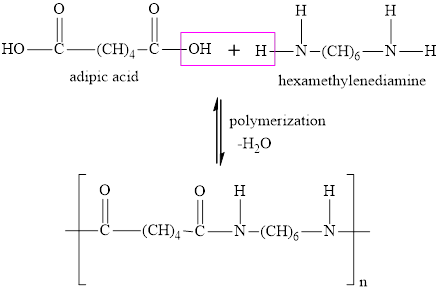
The statement “nylon is an example of a step-growth condensation polymerization” is true.
Want to see more full solutions like this?
Chapter 21 Solutions
Introductory Chemistry: An Active Learning Approach
- Please draw, not just describe!arrow_forwardcan you draw each step on a piece of a paper please this is very confusing to mearrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forward
- Name the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forwardHow to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forward
- Predict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forward
- For the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forwardUsing arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





