
Concept explainers
(a)
Interpretation:
The monosaccharide needs to be classified by determining the type of carbonyl group and number of carbon atoms present in it.
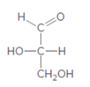
Concept introduction:
Monosaccharide can be characterized according to the type of carbonyl group and the number of carbon atoms in their chain. In the classification of monosaccharide based on type of carbonyl group and number of carbons present in the chain, if the monosaccharide contains an
(b)
Interpretation:
The monosaccharide needs to be classified by determining the type of carbonyl group and number of carbon atoms present in it.
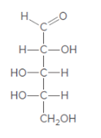
Concept introduction:
Monosaccharide can be characterized according to the type of carbonyl group and the number of carbon atoms in their chain. In the classification of monosaccharide based on type of carbonyl group and number of carbons present in the chain, if the monosaccharide contains an aldehyde group, the prefix aldo- is added to the name and it is called an aldose. On the other hand, if a ketone group is present, it is a ketose or the prefix keto- is added.
(c)
Interpretation:
The monosaccharide needs to be classified by determining the type of carbonyl group and number of carbon atoms present in it.
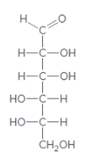
Concept introduction:
Monosaccharide can be characterized according to the type of carbonyl group and the number of carbon atoms in their chain. In the classification of monosaccharide based on type of carbonyl group and number of carbons present in the chain, if the monosaccharide contains an aldehyde group, the prefix aldo- is added to the name and it is called an aldose. On the other hand, if a ketone group is present, it is a ketose or the prefix keto- is added.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- I need help with the following two problems, understanding them in a simple manner. Can you please draw them out for me with a detailed explanation so that I can better comprehend? I'm a visual person, so I definitely need that. Thank you very much!arrow_forwardProblem 54, could you please explain it in detail? Thank you! Step by step, I'm really confused, so please don't make it overly complex. My question is to visually draw it out and demonstrate it to me; I'm confused about that problem, please (not just in words) but demonstrate it to me in all due essence (visually) with descriptions.arrow_forwardExplain the types of electromeric effects +E and -E.arrow_forward
- helpMEarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Drawingarrow_forwardSo, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





