
Concept explainers
(a)
Interpretation:
Whether the given monosaccharide is a D or L sugar needs to be determined.
Concept Introduction:
The configuration stereochemistry of the molecule is represented as D and L enantiomers. In the L isomer of a carbohydrate, the
All the natural sugars are D-isomers.
Answer to Problem 68P
D-isomer
Explanation of Solution
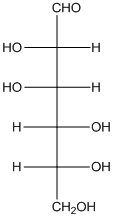
The given monosaccharide is as follows:
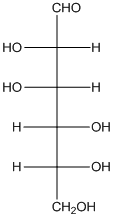
From the structure, the hydroxyl group to the carbon atom away from carbonyl group that is C-5 as hydroxyl group on right hand side thus, it is a D-isomer.
(b)
Interpretation:
The type of carbonyl and number of atoms in the chain of the given monosaccharide needs to be determined.
Concept Introduction:
Monosaccharides are simplest sugar and basic units of carbohydrates. The monosaccharides are further hydrolyzed to form simpler chemical compounds. The general fromula of carbohydrate is
Answer to Problem 68P
Aldehyde group with 6 carbon atom: aldohexose.
Explanation of Solution
The given monosaccharide is as follows:
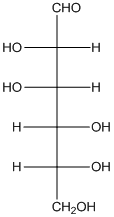
There are 6 carbon atoms present in it and there is an aldehyde group thus, it is an aldohexose.
Thus, the type of carbonyl group is aldehyde and number of atoms in chain is 6 carbon atoms.
(c)
Interpretation:
The enantiomers of the given monosaccharide need to be determined.
Concept Introduction:
The configuration stereochemistry of the molecule is represented as D and L enantiomers. In the L isomer of a carbohydrate, the
Answer to Problem 68P
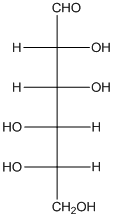
Explanation of Solution
The given monosaccharide is as follows:
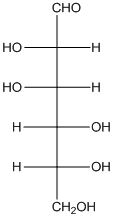
From the structure, the hydroxyl group to the carbon atom away from carbonyl group that is C-5 as hydroxyl group on right hand side thus, it is a D-isomer.
The enantiomer of the given monosaccharide will be L-isomer. The enantiomer of the D-isomer will be non-superimposable mirror image of it. Thus, the structure of L-isomer will be as follows:
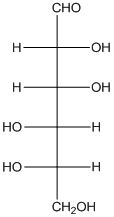
(d)
Interpretation:
The chirality centers needs to be labelled.
Concept Introduction:
The molecules in which there is one or more chiral centers are known as chiral molecules. A carbon attached to four different groups or atom is chiral in nature. The chiral molecules which are mirror images of each other are known as enantiomers. The chiral molecules are non-superimposable to each other. Non-superimposible means one molecule cannot be placed on the other molecule.
Answer to Problem 68P
The 4 chiral centers are represented as follows:
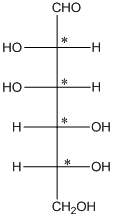
Explanation of Solution
The given monosaccharide is as follows:
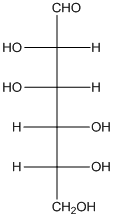
The chiral carbon atom has 4 different groups attached to it. Thus, the labelled carbon atoms are chiral in nature.
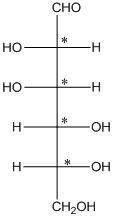
Thus, there are 4 chiral centers in the molecules.
(e)
Interpretation:
The
Concept Introduction:
An epimer is a stereoisomer having difference in configuration at any one chiral center. In the case of anomer, the difference in configuration takes place at the hemiacetal carbon in the cyclic form. The carbon atom is known as anomeric carbon.
Both the
Answer to Problem 68P
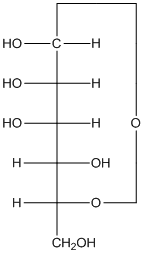
Explanation of Solution
The given monosacchride is as follows:
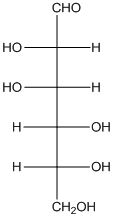
The
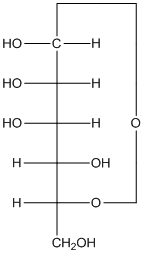
(f)
Interpretation:
The product from the reaction of monosaccharide with Benedict's reagent needs to be determined.
Concept introduction:
Benedict's reagent is used as a mild oxidizing agent and can oxidize aldehyde into the corresponding acid.
Answer to Problem 68P
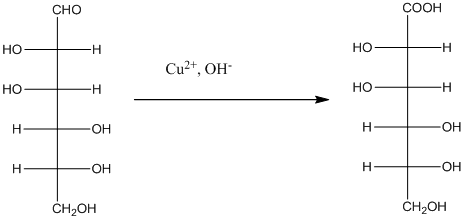
Explanation of Solution
The given monosacchride is as follows:
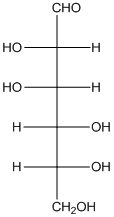
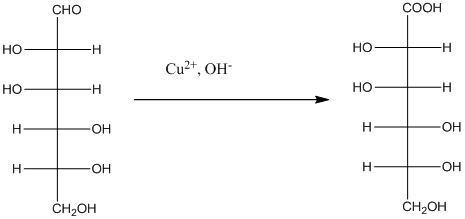
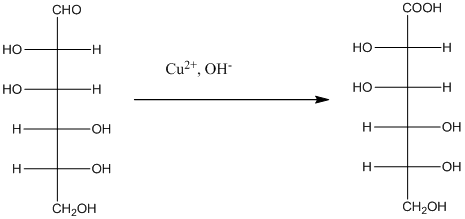
The Benedict's reagent is used as a mild oxidizing agent. Here, Cu2+ complexed with citrate and OH- are present in the Benedict's reagent which is used to identify reducing sugars. It is used as a mild oxidizing agent and can oxidize aldehyde into the corresponding acid. A hydroxyl group is added to the carbon with the carbonyl group when this reagent is added. Cu2+ is converted to Cu+ which then precipitates as Cu2O which is a brick red precipitate.
The reaction is represented as follows:
(g)
Interpretation:
The product formed on reaction of monosaccharide with
Concept introduction:
H2, Pd is used as a reducing agent to reduce compounds like
Answer to Problem 68P
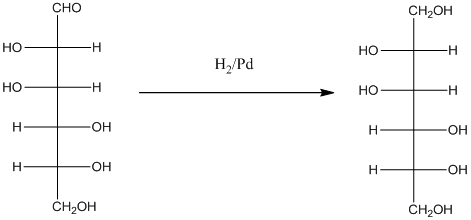
Explanation of Solution
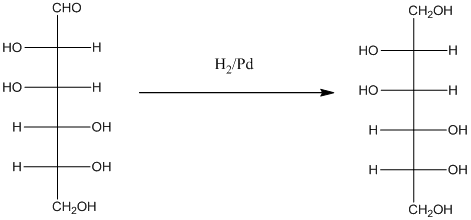
When H2, Pd is added to aldehyde (reducing sugars), the corresponding alcohol is formed. The double bonded oxygen of the aldehyde turns to a hydroxyl group and one hydrogen atom is added to the carbon containing the respective oxygen atom. Pd is the catalyst of this reaction. H2 breaks and gets added as H atoms to oxygen atom and the carbon atom.
The given monosacchride is as follows:
Thus, the reaction with H2, Pd is represented as follows:
(h)
Interpretation:
The monosaccharide needs to be labelled as a reducing or non-reducing sugar.
Concept Introduction:
A reducing sugar is defined as any sugar capable of behaving as a reducing agent having a free aldehyde group or a free ketone group. All monosaccharide are reducing sugars. There are some disaccharides, oligosaccharides and polysaccharides which are also reducing sugars.
Answer to Problem 68P
The given monosaccharide is a reducing sugar.
Explanation of Solution
The given monosaccharide is represented as follows:
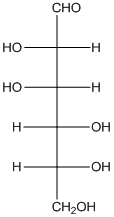
Since, all the monosaccharides are reducing sugar thus, it is also reducing sugar. A reducing sugar acts as a reducing agent. Thus, on reaction with
Want to see more full solutions like this?
Chapter 20 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- State the name and condensed formula of the isothiazole obtained by reacting acetylacetone and thiosemicarbazide.arrow_forwardProvide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardGiven a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forward
- An orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forwardThe molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forwardIn GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forward
- Beer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forwardHow to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forwardGiven a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forward
- Complete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forwardPlease help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





