
Concept explainers
(a)
Interpretation:
Changes in bond order, bond distance, and magnetic properties expected when
Concept Introduction:
Bond order is calculated by the expression given as follows:
The magnetic properties are related to terms such as diamagnetism and paramagnetism. Paramagnetism defines the ability of elements to be weakly attracted in an external magnetic field. It arises due to the presence of unpaired electrons. Diamagnetism defines the ability to be repelled in the external magnetic field environment. This is because diamagnetic species have paired electrons.
The bond length is estimated to be an average of covalent radii of two atoms within a bond. When bond order increases bond becomes stronger and bond length reduces. This accounts for shorter bond length in the case of unsaturated compounds while longer bonds in saturated compounds.
(a)
Explanation of Solution
For
The corresponding molecular orbitals in
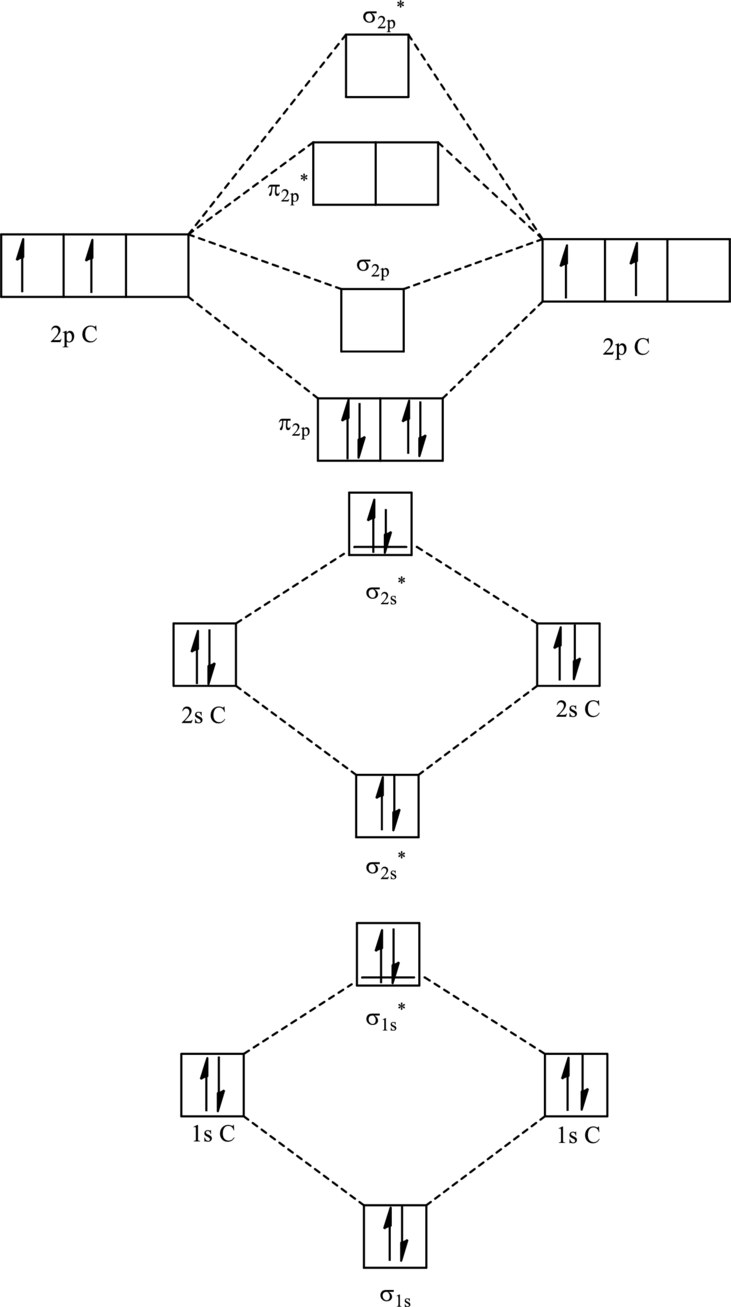
Bond order is calculated by the expression given as follows:
Substitute 4 for anti-bonding electrons and 8 for bonding electrons in equation (1).
Thus bond order is 2 in
For
Substitute 4 for anti-bonding electrons and 7 for bonding electrons in equation (1).
Thus bond order is 1.5 in
Since the reduction in bond implies bond is longer thus the bond length is more in case of
(b)
Interpretation:
Changes in bond order, bond distance, and magnetic properties expected when
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
For
The corresponding molecular orbitals in
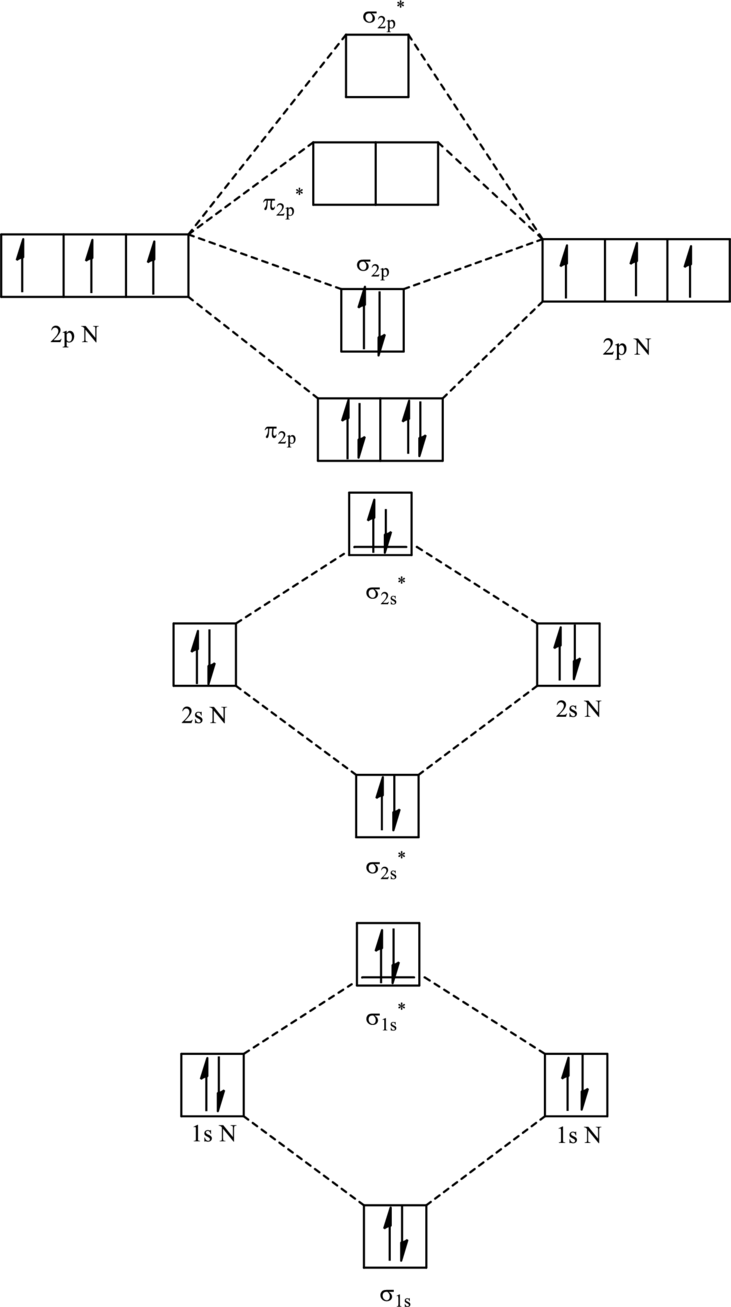
Bond order is calculated by the expression given as follows:
Substitute 4 for anti-bonding electron and 10 for bonding electrons in equation (1).
Thus bond order is 3 in
For
Substitute 4 for anti-bonding electrons and 9 for bonding electrons in equation (1).
Thus bond order is 2.5 in
Since the reduction in bond order implies bond is longer thus the bond length is more in case of
Further in
(b)
Interpretation:
Changes in bond order, bond distance, and magnetic properties expected when
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
For
The corresponding molecular orbitals in
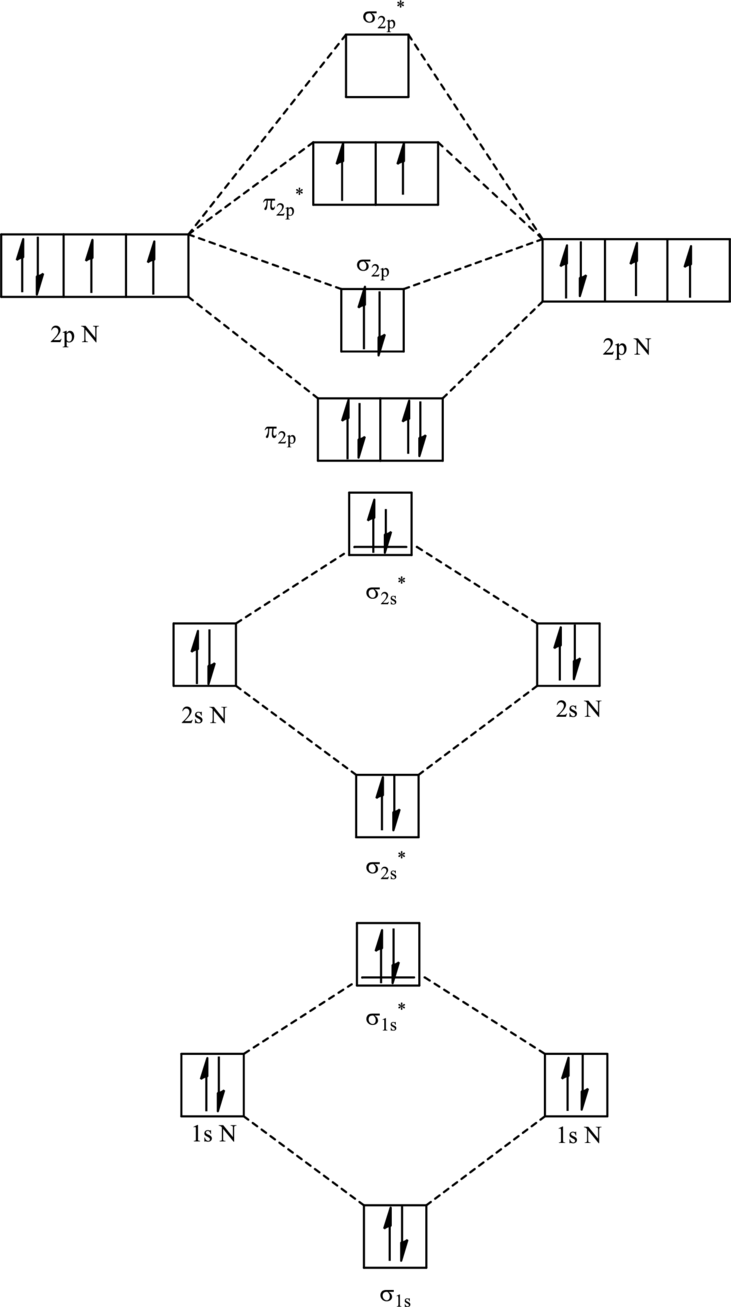
Bond order is calculated by the expression given as follows:
Substitute 6 for anti-bonding electrons and 10 for bonding electrons in equation (1).
Thus bond order is 2 in
For
Substitute 5 for anti-bonding electrons and 10 for bonding electrons in equation (1).
Thus bond order is 2.5 in
Since the reduction in bond order implies bond is shorter thus the bond length is more in case of
Further in
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- COOEt COOEt Step 1 Step 2 Step 3 COOEt COOEt COOH Step 6 OH Step 4 Step 7 (racemic) cyclizes under conditions (8) OTS Step 5 Step 8 ОН OH (racemic) Frontalin (racemic) Shown above are the steps in one of the several published syntheses of Frontalin, a pheromone of the western pine beetle. From the choices provided, show the reagents and conditions by which step 3 of this synthesis might be accomplished. List the reagent(s) in order that will accomplish this transformation. No more than 4 steps are required. List your answer as a single letter (single-step transformation) or a series of letters (multi-step transformation) with no commas separating them. For example, "ab" corresponds to: 1. Eto Na+ 2. NaOH, H₂O NOTE: The order in which you list your letters matters! Reagents: a. Eto Na* g. NaCN b. NaOH, H₂O h. SOCI₂ c. H3O+, heat i. (CH3)2CuLi, ether, -78°C d. LiAlH4 j. H₂O e. p-TsCI, pyridine k. RCO3H f. Br I. H3O+ 1,024arrow_forwardK ← nationa Login - Paymentivet MapQue Draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic acid side product. N 1. excess LiAlH4 2. H₂O ✓ W aarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the alcohol side product. 1. H3O+, heat 2. Neutralizing work-up Drawing Qarrow_forward
- Indicate the procedure (reagent Z) to go from compound A1 to compound A2. A1 Z P(C6H5)3 A2arrow_forwardPlease help with this graph.arrow_forwardogin - PaymentN MapQuest 3 Overview - SAP NetW... Draw the major product of this reaction. Ignore inorganic byproducts. CI 1. NaBH4 2. H₂O C Clever | Portal Job Op Problem Atoms, Bonds and Rings Draw or tap a new bond toarrow_forward
- 2. Draw the remaining two resonance structures for the carbocation intermediate in the meta nitration of methyl benzoate AND explain why the meta orientation is preferred. Hint: how is the placement of the cation favorable after addition of nitronium relative to the electron withdrawing group? (2 pts) H NO2 CO₂Mearrow_forwardLabel all absorptions over 1500 cm-1. Please be specific and mark IR if needed for explanation. Compound OH sp^3 C-H C=O C-O Triglyceridearrow_forwardIdentify the intermediate that is INITIALLY formed in a saponification reaction (hydrolysis of an ester). III -OH H₂O HO OH HO O || A B C III D IV IVarrow_forward
- Help me answer this practice sheet I found for an answer guidearrow_forwardshow the retrosynthesis of this molecule step by step starting with 1,3-dimethoxy benzene H3CO OH OH OCH 3arrow_forwardConsider the reaction of a propanoate ester with hydroxide ion shown below. A series of four alcohol leaving groups were tested to determine which would be the best leaving group. Based on the pKa values of the alcohols, predict which alcohol would produce the fastest hydrolysis reaction. HO FOR A Alcohol I, pKa =16.0 B Alcohol II, pKa =10.0 C Alcohol III, pKa = 7.2 + ROH D Alcohol IV, pKa = 6.6arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




