
Concept explainers
(a)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Lewis structures represent covalent bonds and describe valence electrons configuration of atoms. The covalent bonds are depicted by lines, and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons is estimated.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around the right
symbol of the element to satisfy the octet (or duplet) for each atom. - Add charge on the overall structure in case of polyatomic cation or anion.
The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Here,
(a)
Explanation of Solution
Lewis structure possible for

Since the double and triple bonds can conjugate therefore delocalization occurs that results in various equivalent resonance structures as indicated below:
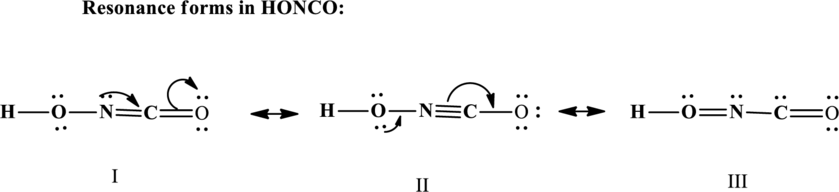
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 5 for
Substitute 5 for
Substitute 4 for
Substitute 5 for
Substitute 4 for
Substitute 6 for
Substitute 6 for
Substitute 6 for
Substitute 1 for
Therefore the non-zero formal charges can be assigned as follows:
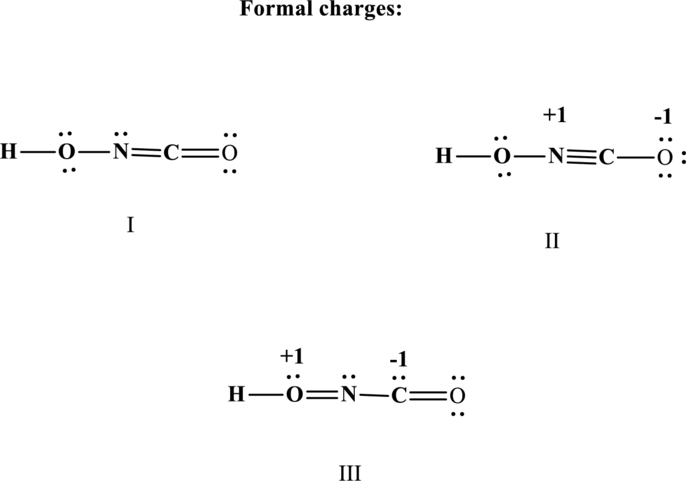
(b)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
Lewis structure possible for
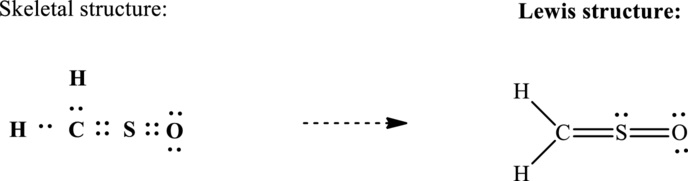
Since the double and triple bonds can conjugate therefore delocalization occurs that results in various equivalent resonance structures as indicated below:
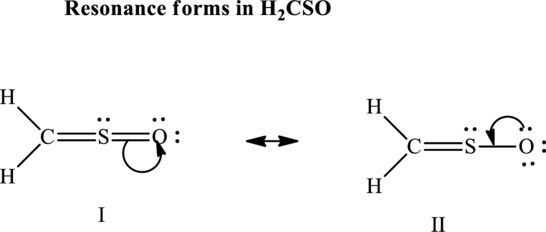
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 6 for
Substitute 6 for
Substitute 6 for
Substitute 6 for
Substitute 1 for
Therefore the formal charges can be assigned as follows:
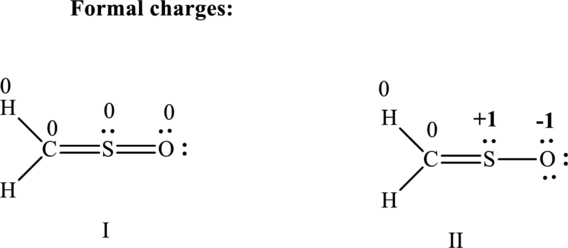
(c)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
Lewis structure for
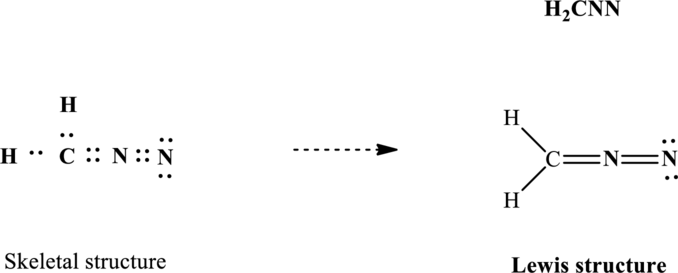
Since the double and triple bonds can conjugate therefore delocalization occurs that results in two equivalent resonance structures as indicated below:
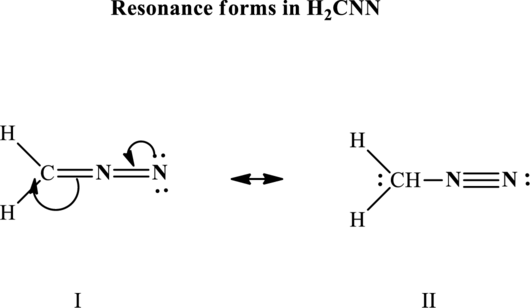
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 5 for
Substitute 5 for
Substitute 5 for
Substitute 5 for
Substitute 4 for
Substitute 1 for
Therefore the formal charges in
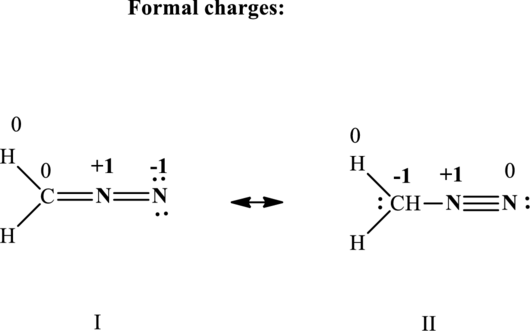
(d)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
Lewis structure for
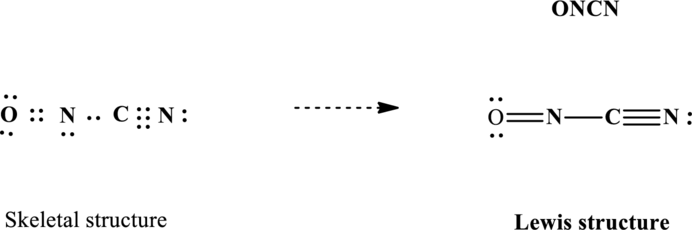
Since the double and triple bonds can conjugate therefore delocalization occurs that results in two equivalent resonance structures as indicated below:
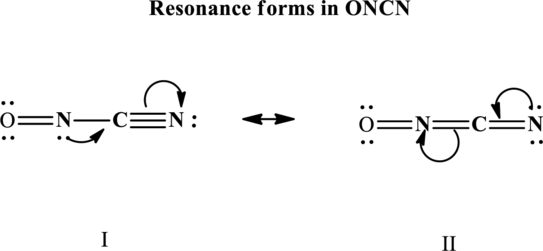
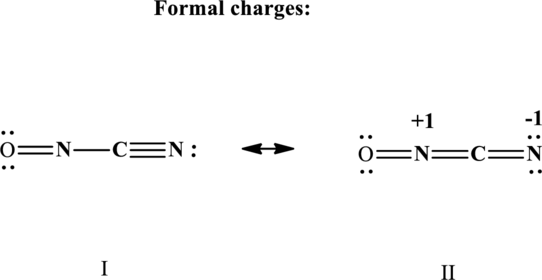
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 5 for
Substitute 5 for
Therefore the non-zero formal charges in
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


