
Concept explainers
(a)
Interpretation:
Lewis structure of
Concept Introduction:
Lewis structures represent covalent bonds and describe valence electrons configuration of atoms. The covalent bonds are depicted by lines and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons are estimated for each atom.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around the
symbol of the element to satisfy the octet (or duplet) for each atom. - Add charge on the overall structure in case of polyatomic cation or anion.
(a)
Explanation of Solution
Hence, 6 pairs are to be allocated to form the Lewis structure of
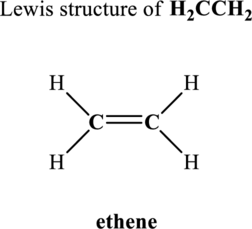
Similarly, total valence electrons is sum of the valence electrons for each atom along with a charge in
Hence, 8 pairs are to be allocated to form the Lewis structure of
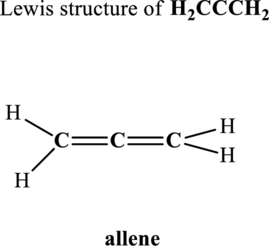
Likewise, total valence electrons in
Hence, 10 pairs are to be allocated to form the Lewis structure of
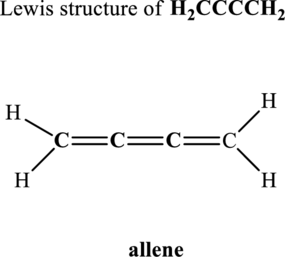
(b)
Interpretation:
The hybridization at each carbon in
Concept Introduction:
The table that relates the steric number with hybridization is as follows:
(b)
Explanation of Solution
In the structure of
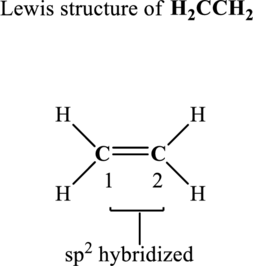
The terminal carbon atoms at positions 1 and 2 are trigonal planar with 3 as steric number hence the hybridization of these carbon atoms is
In the structure of
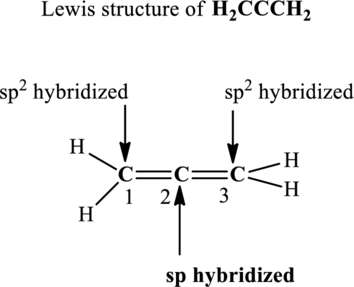
Since the steric number around central carbon is 2 therefore, the hybridization of carbon atom located at positions 2 is
In the structure of
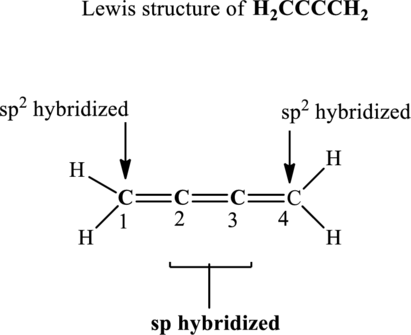
Central carbon atoms have effectively two bond pairs as each double bond is regarded as one single bond pair. Since the steric number around central carbon atoms is 2 therefore, the hybridization of carbon atoms located at positions 2 and 3 is
The terminal carbon atoms at positions 1 and 4 are trigonal planar with 3 as steric number hence the hybridization of this carbon is
(c)
Interpretation:
The type of bonds that connect carbon atoms in
Concept Introduction:
A single bond is always associated with sigma bonds. Molecules that have all atoms connected by sigma bond exclusively are stated to be in
A double bond and carbocation are associated with
A triple bond or central carbon structure similar to allene is always associated with
(c)
Explanation of Solution
In
In
In
(d)
Interpretation:
The bond angles in
Concept Introduction:
Refer to part (c).
(d)
Explanation of Solution
A single bond is always associated with sigma bonds. Molecules that have all atoms connected by sigma bond exclusively are stated to be in
A double bond and carbocation are associated with
A triple bond or central carbon structure similar to allene is always associated with
Thus the bond angles can be illustrated as follows:
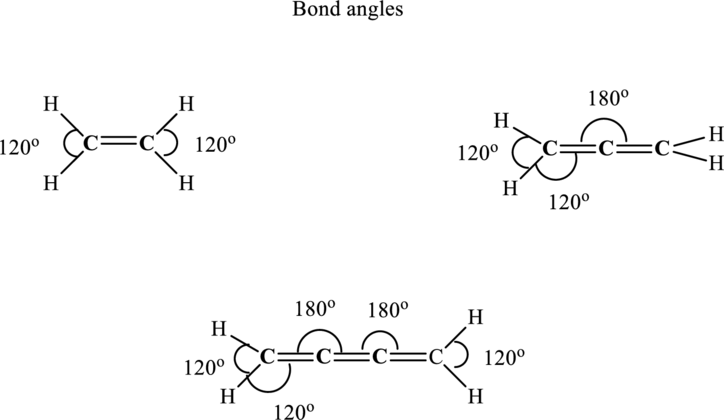
(e)
Interpretation:
Whether all hydrogen atoms lie in the same plane or not in
Concept Introduction:
Refer to part (c).
(e)
Explanation of Solution
In allene, the central carbon that is double bonded to each carbon in different planes is
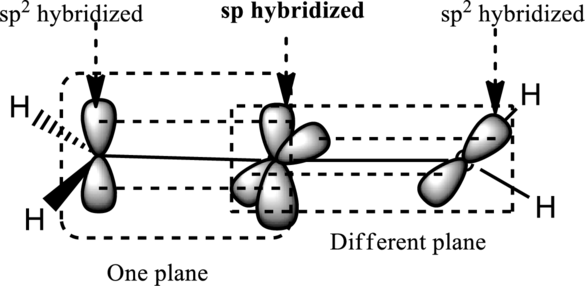
Thus the terminal hydrogen atom on carbon at position 1 is in a different plane than carbon at position 3 in case of
(f)
Interpretation:
Orientation of hydrogen atoms at end of chain has to be determined.
Concept Introduction:
In allene, the central carbon that is double bonded to each carbon in different planes is
(f)
Explanation of Solution
For structures with even number of carbon atoms, for example, consider the structure:
Here, the relative position of the hydrogen atom is indicated as follows:
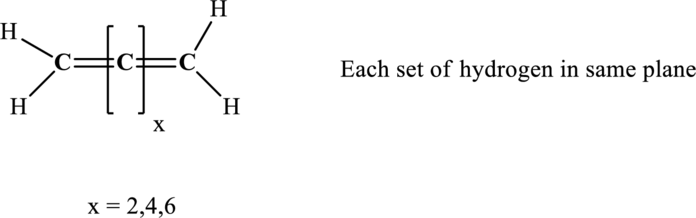
For structures with an odd number of carbon atoms, for example, consider the structure:
Here, the relative position of the hydrogen atom is indicated as follows:

Thus structures with even a number of carbon atoms as in
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- Predict the major products of this organic reaction: H OH 1. LiAlH4 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. G C टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forward
- Predict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forward
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forwardgive example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward
- 2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

