
Concept explainers
Give IUPAC names for the following compounds:
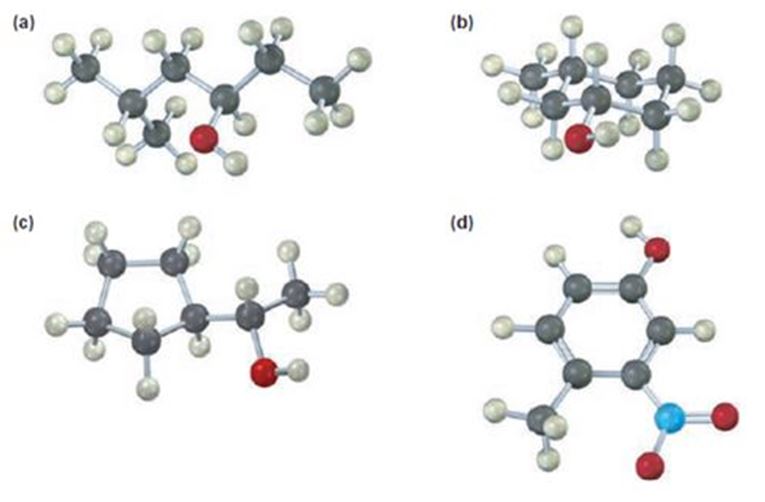
a)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
Alcohols are named as derivatives of the parent alkane using the suffix–ol. The longest carbon chain containing the hydroxyl group is chosen and the parent name is derived by replacing the ending–e with–ol. The akane chain is numbered beginning at the end nearer to the hydroxyl group. The substituents are numbered according to their position on the chain. The name is written listing the substituents in the alphabetical order and indicating the position to which –OH is bonded.
If the compound is chiral then there can be two types of arrangements of the four groups attached to the chiral carbon, R and S. The four groups are arranged in the order of priority as 1,2,3 and 4 following sequence rules. The molecule is viewed by orienting the group of lowest priority (4) points away from the observer. If the arrangement of highest to second highest to third highest is clockwise the R configuration is assigned. If the arrangement is anticlockwise then S configurationis assigned.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
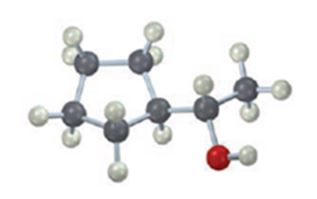
The compound given is
Its IUPAC name is (R) - 5 methylhexan-3-ol.
Explanation of Solution
The name of the compound indicates the presence of a six carbon straight chain with –OH on C3 and a methyl group on C5. Further the molecule is chiral. The groups –OH (highest ranking), -CH2CH(CH3)2(second highest ranking) and -CH2CH3(third highest ranking) are arranged around the chiral carbon in clockwise arrangement. Hence R configuration is assigned.
The compound given is

Its IUPAC name is (R) - 5 methylhexan-3-ol.
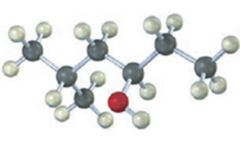
b)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
In naming cyclic alcohols the parent name is derived from the cycloalkene ring by replacing–e of the cycloalkene with–ol. The ring is numbered from the carbon with –OH in such a way that lowest number possible is given to the carbons with substituents.
A cis 1,3-disubstituted cycloalkane has the two substituent groups in a diequatorial arrangement.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
The compound given is
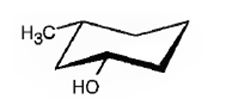
Its IUPAC name is cis-3-methylcyclohexanol.
Explanation of Solution
The structure shows that the compound contains a cyclohexane ring with a –OH and methyl groups in diequatorial positions on C1 and C3. Hence it is a cis-isomer.
The compound given is

Its IUPAC name is cis-3-methylcyclohexanol.
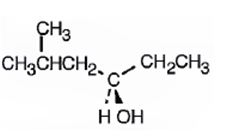
c)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
Alcohols are named as derivatives of the parent alkane using the suffix–ol. The longest carbon chain containing the hydroxyl group is chosen and the parent name is derived by replacing the ending–e with–ol. He akane chain is numbered beginning at the end nearer to the hydroxyl group. The substituents are numbered according to their position on the chain. The name is written listing the substituents in the alphabetical order and indicating the position to which –OH is bonded.
If the compound is chiral then there can be two types of arrangements of the four groups attached to the chiral carbon, R and S. The four groups are arranged in the order of priority as 1,2,3 and 4 following sequence rules. The molecule is viewed by orienting the group of lowest priority (4) points away from the observer. If the arrangement of highest to second highest to third highest is clockwise the R configuration is assigned. If the arrangement is anticlockwise then S configurationis assigned.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
The compound given is
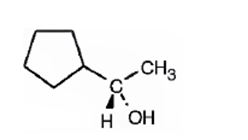
Its IUPAC name is (S)-1-cyclopentylethanol.
Explanation of Solution
The structure shows that the compound contains a two carbon straight chain with –OH and a cyclopentyl groups on C1.
The four groups, -OH(Highest priority), Cyclopentyl (second highest priority), methyl (third highest priority) attached to C1 are arranged anticlockwise when viewed away from hydrogen (fourth priority). Hence S configuration is assigned.
The compound given is

Its IUPAC name is (S)-1-cyclopentylethanol.
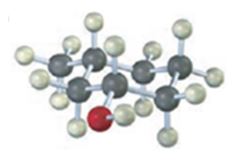
d)
Interpretation:
To give the IUPAC name of the compound given.
Concept introduction:
Hydroxybenzenes are named as derivatives of phenol. If the ring contains other substituent also the ring is numbered from the carbon with –OH in such a way that lowest number possible is given to the carbon with the substituent. While writing the name the substituents are arranged in the alphabetical order.
To give:
The IUPAC name of the compound given.
Answer to Problem 20VC
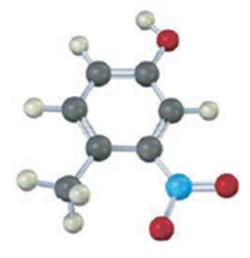
The compound given is.
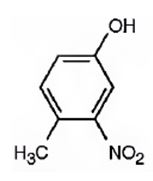
Its IUPAC name is 4-methyl-3-nitrophenol.
Explanation of Solution
The structure of the compound shows a benzene ring with –OH on C1, a nitro group on C3 and a methyl group on C4.
The compound given is.

Its IUPAC name is 4-methyl-3-nitrophenol.
Want to see more full solutions like this?
Chapter 17 Solutions
Organic Chemistry
- १ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forwardDraw the molecules.arrow_forwardDraw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward
- . Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forwardDraw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward
- [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPlease draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forward
- An open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forwardConsider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forwardTo improve chromatographic separation, you must: Increase the number of theoretical plates on the column. Increase the height of theoretical plates on the column. Increase both the number and height of theoretical plates on the column. Increasing the flow rate of the mobile phase would Increase longitudinal diffusion Increase broadening due to mass transfer Increase broadening due to multiple paths You can improve the separation of components in gas chromatography by: Rasing the temperature of the injection port Rasing the temperature of the column isothermally Rasing the temperature of the column using temperature programming In GC, separation between two different solutes occurs because the solutes have different solubilities in the mobile phase the solutes volatilize at different rates in the injector the solutes spend different amounts of time in the stationary phasearrow_forward

 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



