
Concept explainers
(a)
Interpretation:
Structure of
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between  group present in carboxylic acid and
group present in carboxylic acid and 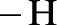 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
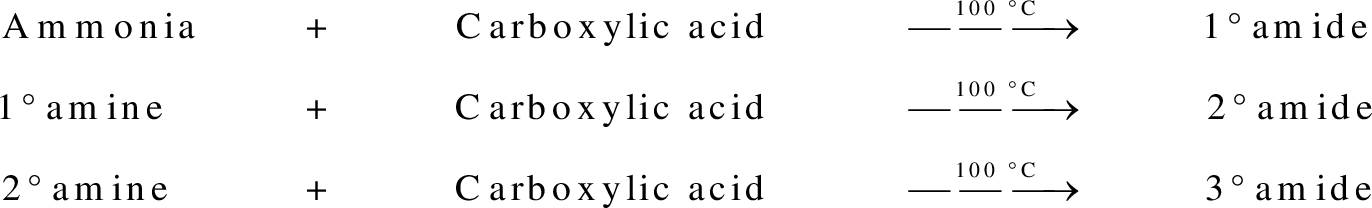
(b)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the  group present in carboxylic acid and
group present in carboxylic acid and 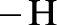 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
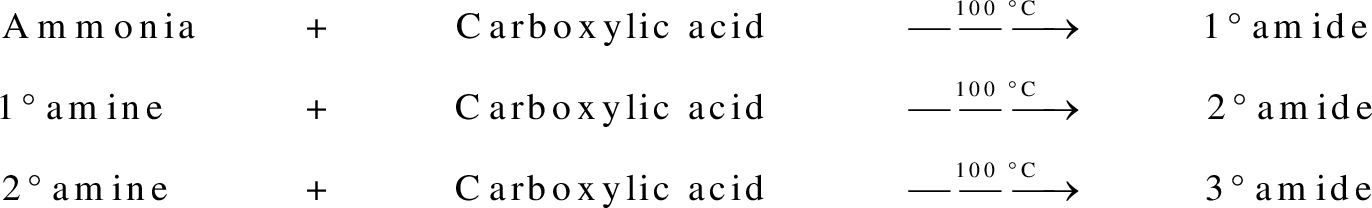
(c)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the  group present in carboxylic acid and
group present in carboxylic acid and 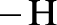 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
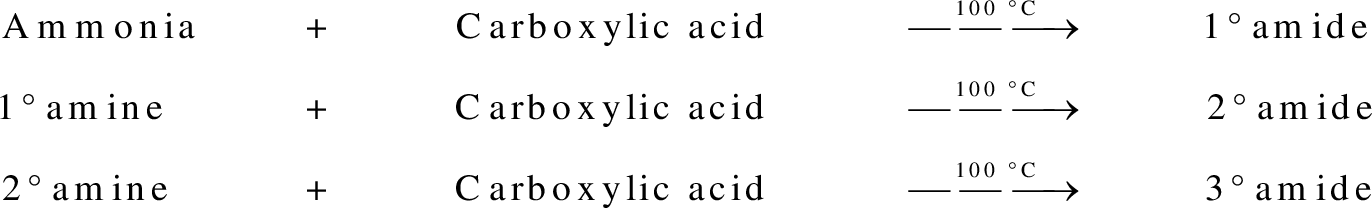
(d)
Interpretation:
Structure of carboxylic acid that is required to produce the given compound as product through amidification has to be given.
Concept Introduction:
Amides are synthesized using amidification reaction. This involves a reaction between amine and carboxylic acid. In this reaction, the  group present in carboxylic acid and
group present in carboxylic acid and 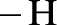 from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
from ammonia or amine is lost to give amide as the product. Water is obtained as a by-product in this reaction. The general reaction scheme for synthesis of amides can be given as,
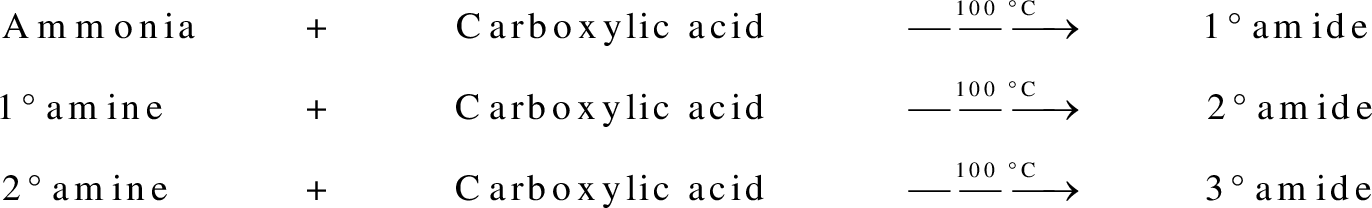
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



