
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.9P
Which
a. 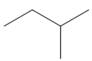 b.
b. 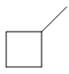 c.
c. 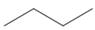
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used Ai solution
Don't used Ai solution
Please correct answer and don't used hand raiting
Chapter 15 Solutions
Organic Chemistry-Package(Custom)
Ch. 15 - Prob. 15.1PCh. 15 - Prob. 15.2PCh. 15 - Draw the product formed when a chlorine atom (Cl)...Ch. 15 - Prob. 15.4PCh. 15 - Prob. 15.5PCh. 15 - Problem 15.6 Using mechanism 15.1 as guide, write...Ch. 15 - Calculate m0 for the two propagation steps in the...Ch. 15 - Prob. 15.8PCh. 15 - Problem 15.8 Which bond in the each compound is...Ch. 15 - Prob. 15.10P
Ch. 15 - Prob. 15.11PCh. 15 - Synthesize each compound from (CH3)3CH. a....Ch. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - Prob. 15.15PCh. 15 - Prob. 15.16PCh. 15 - Prob. 15.17PCh. 15 - Prob. 15.18PCh. 15 - Draw all constitutional isomers formed when each...Ch. 15 - Draw the structure of the four allylic halides...Ch. 15 - Which compounds can be prepared in good yield by...Ch. 15 - Which CH bond is most readily cleaved in linolenic...Ch. 15 - Prob. 15.23PCh. 15 - Draw the products formed when each alkene is...Ch. 15 - Problem 15.24 When adds to under radical...Ch. 15 - Prob. 15.26PCh. 15 - Draw an energy diagram for the two propagation...Ch. 15 - Prob. 15.28PCh. 15 - Problem 15.27 Draw the steps of the mechanism that...Ch. 15 - Prob. 15.30PCh. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Why is a benzylic CH bond labeled in red unusually...Ch. 15 - Prob. 15.35PCh. 15 - Prob. 15.36PCh. 15 - Prob. 15.37PCh. 15 - Prob. 15.38PCh. 15 - What alkane is needed to make each alkyl halide by...Ch. 15 - Which alkyl halides can be prepared in good yield...Ch. 15 - Prob. 15.41PCh. 15 - 15.40 Explain why radical bromination of p-xylene...Ch. 15 - a. What product(s) (excluding stereoisomers) are...Ch. 15 - Prob. 15.44PCh. 15 - Prob. 15.45PCh. 15 - Prob. 15.46PCh. 15 - 15.44 Draw all constitutional isomers formed when...Ch. 15 - Draw the organic products formed in each reaction....Ch. 15 - Prob. 15.49PCh. 15 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 15 - Prob. 15.51PCh. 15 - Prob. 15.52PCh. 15 - Prob. 15.53PCh. 15 - Prob. 15.54PCh. 15 - 15.53 Consider the following bromination: .
a....Ch. 15 - 15.54 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 15.57PCh. 15 - An alternative mechanism for the propagation steps...Ch. 15 - Prob. 15.59PCh. 15 - Prob. 15.60PCh. 15 - Devise a synthesis of each compound from...Ch. 15 - Devise a synthesis of each target compound from...Ch. 15 - Devisea synthesis of each target compound from the...Ch. 15 - Devise a synthesis of each compound using CH3CH3...Ch. 15 - Prob. 15.65PCh. 15 - 15.63 As described in Section 9.16, the...Ch. 15 - 15.64 Ethers are oxidized with to form...Ch. 15 - Prob. 15.68PCh. 15 - Prob. 15.69PCh. 15 - 15.67 In cells, vitamin C exists largely as its...Ch. 15 - What monomer is needed to form each...Ch. 15 - Prob. 15.72PCh. 15 - Prob. 15.73PCh. 15 - 15.71 Draw a stepwise mechanism for the following...Ch. 15 - 15.72 As we will learn in Chapter 30, styrene...Ch. 15 - Prob. 15.76PCh. 15 - 15.74 A and B, isomers of molecular formula , are...Ch. 15 - Prob. 15.78PCh. 15 - Radical chlorination of CH3CH3 forms two minor...Ch. 15 - 15.76 Draw a stepwise mechanism for the...Ch. 15 - Prob. 15.81PCh. 15 - Prob. 15.82PCh. 15 - Prob. 15.83P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (12) Which one of the following statements about fluo- rometry is FALSE? a) Fluorescence is better detected at 90 from the exci- tation direction. b) Fluorescence is typically shifted to longer wave- length from the excitation wavelength. c) For most fluorescent compounds, radiation is pro- duced by a transitionarrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardIndicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
- 1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY