
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.70P
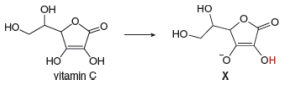
In cells, vitamin C exists largely as its conjugate base X. X is an antioxidant because radicals formed in oxidation processes abstract the labeled H atom, forming a new radical that halts oxidation. Draw the structure of the radical formed by H abstraction, and explain why this H atom is most easily removed.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
8:57 PM Sun Jan 26
Content
←
Explanation Page
X Content
X
ALEKS Jade Nicol - Le
A https://www-av
C
www-awa.aleks.com
O States of Matter
Understanding consequences of important physical properties of liquids
? QUESTION
Liquid A is known to have a lower viscosity and lower surface tension than Liquid B.
Use these facts to predict the result of each experiment in the table below, if you can.
experiment
Liquid A and Liquid B are each pumped
through tubes with an inside diameter of
27.0 mm, and the pressures PA and PB
needed to produce a steady flow of
2.4 mL/s are measured.
25.0 mL of Liquid A are poured into a
beaker, and 25.0 mL of Liquid B are poured
into an identical beaker. Stirrers in each
beaker are connected to motors, and the
forces FA and FB needed to stir each liquid
at a constant rate are measured.
predicted outcome
OPA will be greater than PB
OPA will be less than PB
OPA will be equal to PB
It's impossible to predict whether PA or PB will
be greater without more information.…
Show work. Don't give Ai generated solution
5. Please draw in the blanks the missing transition states and the correlated products. Explicitly
display relevant absolute stereochemical configuration.
MeOH
I
OMe
H
Endo transition state,
dienophile approaching from the bottom of diene
+
H
ཎྞཾ ཌཱརཱ༔,_o
OMe
H
H
OMe
Endo transition state,
dienophile approaching from the top of diene or
from the bottom but horizontally flipped (draw one)
+
Exo transition state,
dienophile approaching from the top of diene or
from the bottom but horizontally flipped (draw one)
Exo transition state,
dienophile approaching from the top of diene or
from the bottom but horizontally flipped (draw one)
MeO H
H
MeO H
MeO H
MeO H
H
Chapter 15 Solutions
Organic Chemistry-Package(Custom)
Ch. 15 - Prob. 15.1PCh. 15 - Prob. 15.2PCh. 15 - Draw the product formed when a chlorine atom (Cl)...Ch. 15 - Prob. 15.4PCh. 15 - Prob. 15.5PCh. 15 - Problem 15.6 Using mechanism 15.1 as guide, write...Ch. 15 - Calculate m0 for the two propagation steps in the...Ch. 15 - Prob. 15.8PCh. 15 - Problem 15.8 Which bond in the each compound is...Ch. 15 - Prob. 15.10P
Ch. 15 - Prob. 15.11PCh. 15 - Synthesize each compound from (CH3)3CH. a....Ch. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - Prob. 15.15PCh. 15 - Prob. 15.16PCh. 15 - Prob. 15.17PCh. 15 - Prob. 15.18PCh. 15 - Draw all constitutional isomers formed when each...Ch. 15 - Draw the structure of the four allylic halides...Ch. 15 - Which compounds can be prepared in good yield by...Ch. 15 - Which CH bond is most readily cleaved in linolenic...Ch. 15 - Prob. 15.23PCh. 15 - Draw the products formed when each alkene is...Ch. 15 - Problem 15.24 When adds to under radical...Ch. 15 - Prob. 15.26PCh. 15 - Draw an energy diagram for the two propagation...Ch. 15 - Prob. 15.28PCh. 15 - Problem 15.27 Draw the steps of the mechanism that...Ch. 15 - Prob. 15.30PCh. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Why is a benzylic CH bond labeled in red unusually...Ch. 15 - Prob. 15.35PCh. 15 - Prob. 15.36PCh. 15 - Prob. 15.37PCh. 15 - Prob. 15.38PCh. 15 - What alkane is needed to make each alkyl halide by...Ch. 15 - Which alkyl halides can be prepared in good yield...Ch. 15 - Prob. 15.41PCh. 15 - 15.40 Explain why radical bromination of p-xylene...Ch. 15 - a. What product(s) (excluding stereoisomers) are...Ch. 15 - Prob. 15.44PCh. 15 - Prob. 15.45PCh. 15 - Prob. 15.46PCh. 15 - 15.44 Draw all constitutional isomers formed when...Ch. 15 - Draw the organic products formed in each reaction....Ch. 15 - Prob. 15.49PCh. 15 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 15 - Prob. 15.51PCh. 15 - Prob. 15.52PCh. 15 - Prob. 15.53PCh. 15 - Prob. 15.54PCh. 15 - 15.53 Consider the following bromination: .
a....Ch. 15 - 15.54 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 15.57PCh. 15 - An alternative mechanism for the propagation steps...Ch. 15 - Prob. 15.59PCh. 15 - Prob. 15.60PCh. 15 - Devise a synthesis of each compound from...Ch. 15 - Devise a synthesis of each target compound from...Ch. 15 - Devisea synthesis of each target compound from the...Ch. 15 - Devise a synthesis of each compound using CH3CH3...Ch. 15 - Prob. 15.65PCh. 15 - 15.63 As described in Section 9.16, the...Ch. 15 - 15.64 Ethers are oxidized with to form...Ch. 15 - Prob. 15.68PCh. 15 - Prob. 15.69PCh. 15 - 15.67 In cells, vitamin C exists largely as its...Ch. 15 - What monomer is needed to form each...Ch. 15 - Prob. 15.72PCh. 15 - Prob. 15.73PCh. 15 - 15.71 Draw a stepwise mechanism for the following...Ch. 15 - 15.72 As we will learn in Chapter 30, styrene...Ch. 15 - Prob. 15.76PCh. 15 - 15.74 A and B, isomers of molecular formula , are...Ch. 15 - Prob. 15.78PCh. 15 - Radical chlorination of CH3CH3 forms two minor...Ch. 15 - 15.76 Draw a stepwise mechanism for the...Ch. 15 - Prob. 15.81PCh. 15 - Prob. 15.82PCh. 15 - Prob. 15.83P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H H (1) H C. C C .H (2) (3) Cl H The ideal value for bond angle (1) is (Choose one) and the ideal value for bond angle (3) is (Choose one) degrees, the value for bond angle (2) is (Choose one) degrees, degrees.arrow_forwardShow work.....don't give Ai generated solutionarrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- 10. Complete the following halogenation reactions for alkanes. Draw the structures of one of the many possible products for each reaction. Name the reactant and product. a) CH₂- CH-CH2-CH3 + Br₂ CH₂ UV UV b) + Cl2 c) CH3-CH₂ CHICHCHICH-CH CH₂-CH₂ + F2 UVarrow_forwardWhich of the following processes involves the largest photon energy? Group of answer choices Electron promotion from n=2 to n=5 Electron relaxing from n=4 to n=3 Ionization of an electron from n=2 Ionization of an electron from n=4arrow_forwardWhich of the following compounds does not match atomic ratio expectations in Mendeleev's 1872 periodic table? Group of answer choices NO2 Al2O3 SO3 CaOarrow_forward
- Need help with 14 and 15. 14. bromobenzene + (CHs),CuLi + THF / -78° followed by water quench is a. toluene else!! b. xylene c. cumene d. styrene e. something 15. When cumene + H,SO, / Na,Cr, 0,/water are mixed (refluxed) what is produced? a. 2-phenylpropanol phenol e. styrene b. benzoic acid c. no reaction!arrow_forwardWhich of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.) Group of answer choices px dxz dx2-y2 py dxy sarrow_forwardWhich of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices Li-Na-K Al-Ga-In Cr-Mo-W K-Rb-Csarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY