
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.34P
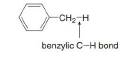
Why is a benzylic
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A small artisanal cheesemaker is testing the acidity of their milk
before it coagulates. During fermentation, bacteria produce lactic
acid (K₁ = 1.4 x 104), a weak acid that helps to curdle the milk and
develop flavor. The cheesemaker has measured that the developing
mixture contains lactic acid at an initial concentration of 0.025 M.
Your task is to calculate the pH of this mixture and determine whether
it meets the required acidity for proper cheese development. To
achieve the best flavor, texture and reduce/control microbial growth,
the pH range needs to be between pH 4.6 and 5.0.
Assumptions:
Lactic acid is a monoprotic acid
H
H
:0:0:
H-C-C
H
:0:
O-H
Figure 1: Lewis Structure for Lactic Acid
For simplicity, you can use the generic formula HA to represent the acid
You can assume lactic acid dissociation is in water as milk is mostly water.
Temperature is 25°C
1. Write the K, expression for the dissociation of lactic acid in the space provided. Do not forget to
include state symbols.…
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product
structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s).
Be sure to account for all bond-breaking and bond-making steps.
:0:
:0
H.
0:0
:0:
:6:
S:
:0:
Select to Edit Arrows
::0
Select to Edit Arrows
H
:0:
H
:CI:
Rotation
Select to Edit Arrows
H.
<
:0:
:0:
:0:
S:
3:48 PM Fri Apr 4
K
Problem 4 of 10
Submit
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product
structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s).
Be sure to account for all bond-breaking and bond-making steps.
Mg.
:0:
Select to Add Arrows
:0:
:Br:
Mg
:0:
:0:
Select to Add Arrows
Mg.
Br:
:0:
0:0-
Br
-190
H
0:0
Select to Add Arrows
Select to Add Arrows
neutralizing workup
H
CH3
Chapter 15 Solutions
Organic Chemistry-Package(Custom)
Ch. 15 - Prob. 15.1PCh. 15 - Prob. 15.2PCh. 15 - Draw the product formed when a chlorine atom (Cl)...Ch. 15 - Prob. 15.4PCh. 15 - Prob. 15.5PCh. 15 - Problem 15.6 Using mechanism 15.1 as guide, write...Ch. 15 - Calculate m0 for the two propagation steps in the...Ch. 15 - Prob. 15.8PCh. 15 - Problem 15.8 Which bond in the each compound is...Ch. 15 - Prob. 15.10P
Ch. 15 - Prob. 15.11PCh. 15 - Synthesize each compound from (CH3)3CH. a....Ch. 15 - Prob. 15.13PCh. 15 - Prob. 15.14PCh. 15 - Prob. 15.15PCh. 15 - Prob. 15.16PCh. 15 - Prob. 15.17PCh. 15 - Prob. 15.18PCh. 15 - Draw all constitutional isomers formed when each...Ch. 15 - Draw the structure of the four allylic halides...Ch. 15 - Which compounds can be prepared in good yield by...Ch. 15 - Which CH bond is most readily cleaved in linolenic...Ch. 15 - Prob. 15.23PCh. 15 - Draw the products formed when each alkene is...Ch. 15 - Problem 15.24 When adds to under radical...Ch. 15 - Prob. 15.26PCh. 15 - Draw an energy diagram for the two propagation...Ch. 15 - Prob. 15.28PCh. 15 - Problem 15.27 Draw the steps of the mechanism that...Ch. 15 - Prob. 15.30PCh. 15 - Prob. 15.31PCh. 15 - Prob. 15.32PCh. 15 - Prob. 15.33PCh. 15 - Why is a benzylic CH bond labeled in red unusually...Ch. 15 - Prob. 15.35PCh. 15 - Prob. 15.36PCh. 15 - Prob. 15.37PCh. 15 - Prob. 15.38PCh. 15 - What alkane is needed to make each alkyl halide by...Ch. 15 - Which alkyl halides can be prepared in good yield...Ch. 15 - Prob. 15.41PCh. 15 - 15.40 Explain why radical bromination of p-xylene...Ch. 15 - a. What product(s) (excluding stereoisomers) are...Ch. 15 - Prob. 15.44PCh. 15 - Prob. 15.45PCh. 15 - Prob. 15.46PCh. 15 - 15.44 Draw all constitutional isomers formed when...Ch. 15 - Draw the organic products formed in each reaction....Ch. 15 - Prob. 15.49PCh. 15 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 15 - Prob. 15.51PCh. 15 - Prob. 15.52PCh. 15 - Prob. 15.53PCh. 15 - Prob. 15.54PCh. 15 - 15.53 Consider the following bromination: .
a....Ch. 15 - 15.54 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 15.57PCh. 15 - An alternative mechanism for the propagation steps...Ch. 15 - Prob. 15.59PCh. 15 - Prob. 15.60PCh. 15 - Devise a synthesis of each compound from...Ch. 15 - Devise a synthesis of each target compound from...Ch. 15 - Devisea synthesis of each target compound from the...Ch. 15 - Devise a synthesis of each compound using CH3CH3...Ch. 15 - Prob. 15.65PCh. 15 - 15.63 As described in Section 9.16, the...Ch. 15 - 15.64 Ethers are oxidized with to form...Ch. 15 - Prob. 15.68PCh. 15 - Prob. 15.69PCh. 15 - 15.67 In cells, vitamin C exists largely as its...Ch. 15 - What monomer is needed to form each...Ch. 15 - Prob. 15.72PCh. 15 - Prob. 15.73PCh. 15 - 15.71 Draw a stepwise mechanism for the following...Ch. 15 - 15.72 As we will learn in Chapter 30, styrene...Ch. 15 - Prob. 15.76PCh. 15 - 15.74 A and B, isomers of molecular formula , are...Ch. 15 - Prob. 15.78PCh. 15 - Radical chlorination of CH3CH3 forms two minor...Ch. 15 - 15.76 Draw a stepwise mechanism for the...Ch. 15 - Prob. 15.81PCh. 15 - Prob. 15.82PCh. 15 - Prob. 15.83P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Iarrow_forwardDraw the Markovnikov product of the hydrobromination of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. + Explanation Check 1 X E 4 1 1 1 1 1 HBr Click and drag to start drawing a structure. 80 LE #3 @ 2 $4 0 I அ2 % 85 F * K M ? BH 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center & 6 27 FG F10 8 9 R T Y U D F G H P J K L Z X C V B N M Q W A S H option command H command optiarrow_forwardBe sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. Predict the major products of the following reaction. Explanation Q F1 A Check F2 @ 2 # 3 + X 80 F3 W E S D $ 4 I O H. H₂ 2 R Pt % 05 LL ee F6 F5 T <6 G Click and drag to start drawing a structure. 27 & A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Acce Y U H DII 8 9 F10 4 J K L Z X C V B N M T H option command F11 P H commandarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. H :0: CH3 O: OH Q CH3OH2+ Draw Intermediate protonation CH3OH CH3OH nucleophilic addition H Draw Intermediate deprotonation :0: H3C CH3OH2* protonation H 0: H CH3 H.arrow_forwardPredicting the reactants or products of hemiacetal and acetal formation uentify the missing organic reactants in the following reaction: H+ X+Y OH H+ за Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. ? olo 18 Ar © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardcan someone please answer thisarrow_forward
- Please, please help me figure out the the moles, molarity and Ksp column. Step by step details because I've came up with about three different number and have no idea what I'm doing wrong.arrow_forwardwhat reagents are used to get this product from this reactant? Br OCH3arrow_forwardcan someone answer this pleasearrow_forward
- can someone do the reaction mechanism for this reaction and draw the molecules for Q2 and q3arrow_forwardIn this question, the product of the aldol condensation is shown. What would be the reactants for this product? Please provide a detailed explanation, as well as a drawing showing how the reactants will react to produce the product.arrow_forward7. Propene undergoes a hydration reaction with water in the presence of an acid. a. There are two possible products for this reaction, both with the formula C,H,O. Show their structural formulas and names. (A1, B2) SCH4UR Name: (answer for part a. here!) VER 3 2021-2022 b. Which of the two products do you predict will form. Explain your choice using details from your learning. (B3)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY