
Concept explainers
Interpretation :
Correct option must be chosen regarding the temperature and pressure at which solid graphite, solid diamond and liquid carbon coexists.
Concept Introduction :
Phase diagram gives the idea of different phases at different temperature and pressure. In the phase diagram of carbon, carbon can exit in solid diamond, solid graphite, liquid carbon and vapor phase. There are two triples points; in one point diamond, graphite and liquid carbon exist and in other point graphite liquid and vapor exist.
Answer to Problem 7STP
Correct answer: From the triple point, no option is correct as the point corresponds to
Explanation of Solution
Reason for correct option: The given phase diagram of carbon is represented as follows:
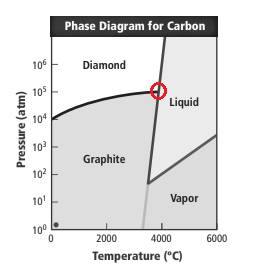
The point marked is triple point where three phases that is diamond, liquid and graphite exist. From the graph it can be seen that this point is at
Thus, none of the option is correct.
Reasons for incorrect options:
In option (a), the pressure and temperature is
In option (b), the pressure and temperature is
In option (c), the pressure and temperature is
In option (d), the pressure and temperature is 80 atm and 3500 K respectively. This cannot be possible as at this low pressure diamond will not exist.
Chapter 12 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Introductory Chemistry (6th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Campbell Biology (11th Edition)
Anatomy & Physiology (6th Edition)
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





