
Concept explainers
(a)
Interpretation:
The number of particles in 1 unit cell for simple cubic needs to be determined.
Concept introduction:
In simple cubic unit cell, each atom in the lattice has 6 neighboring atoms arranged in octahedral arrangement. It is an inefficient way to pack atoms in the given space. In simple cubic lattice, 52% of the total space is occupied by atoms. The example is NaCl in which each cation is surrounded by 6 anions and vice versa.
Answer to Problem 70A
8
Explanation of Solution
In simple unit cell, there are 8 atoms at corners sharing 1/8th of the original volume of the cube.
The number of atoms at centers and faces is equal to zero. Therefore, the total number of atoms per unit cell will be 8 that is present on 8 corners of the lattice.
Thus, the number of particles per unit cell is 8.
(b)
Interpretation:
The number of particles in 1 unit cell for body-centered cubic needs to be determined.
Concept introduction:
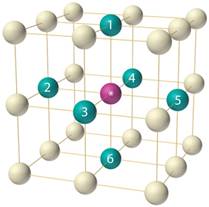
In body-centered cubic unit cell, the spheres are packed together more efficiently. There are 8 nearest neighbors in the unit cell and 68% of the volume is occupied by atoms.
Answer to Problem 70A
9
Explanation of Solution
In a simple unit cell, there are 8 atoms at the corners sharing 1/8th of the original volume of the cube.
The number of atoms at centers and faces is equal to 1 and zero respectively. Therefore, the total number of atoms per unit cell will be 9 which includes 8 atoms present at 8 corners of the lattice and 1 at the center.
Thus, the number of particles per unit cell is 9 that is one more than simple cubic.
Chapter 12 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Introductory Chemistry (6th Edition)
Chemistry: Structure and Properties (2nd Edition)
Brock Biology of Microorganisms (15th Edition)
Biology: Life on Earth (11th Edition)
Campbell Biology (11th Edition)
- First image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forwardwhat is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forwardOne step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forward
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





