
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 16P
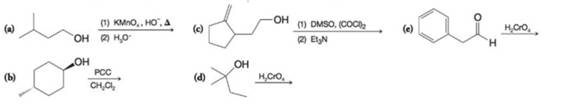
Predict the organic product from each of the following oxidation reactions.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
H2(g) + I2(g) ⇔ 2HI(g)
Using the above equilibrium, find the equilibrium concentration of H2 if the intial concentration of both H2 and I2 are 2.0. K at this temperature is 55.64.
find K, the equilibrium constant, if the inital concentration of SO3 is 0.166 M, and the equilibrium concentration of O2 is 0.075 M.
2SO3 (g) ⇌ 2SO2 (g) + O2 (g)
Chapter 12 Solutions
Organic Chemistry
Ch. 12 - Prob. 1PPCh. 12 - Prob. 2PPCh. 12 - Prob. 3PPCh. 12 - PRACTICE PROBLEM 12.4 Predict the products of the...Ch. 12 - Prob. 5PPCh. 12 - Prob. 6PPCh. 12 - Practice Problem 12.7
Provide retrosynthetic...Ch. 12 - Prob. 8PPCh. 12 - What products would you expect from the reaction...Ch. 12 - What products would you expect from the reaction...
Ch. 12 - What product (or products) would be formed from...Ch. 12 - Prob. 12PCh. 12 - 12.13 Write reaction conditions and the product...Ch. 12 - Prob. 14PCh. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the organic product from each of the...Ch. 12 - Predict the major organic product from each of the...Ch. 12 - Synthesize each of the following compounds from...Ch. 12 - Prob. 20PCh. 12 - 21. Write a mechanism for the following reaction....Ch. 12 - Prob. 22PCh. 12 - 23. What organic products A-H would you expect...Ch. 12 - Prob. 24PCh. 12 - Show how 1-pentanol could be transformed into each...Ch. 12 - Provide the reagents needed to accomplish...Ch. 12 - Prob. 27PCh. 12 - For each of the following alcohols, write a...Ch. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Predict the major organic product from each of the...Ch. 12 - 34. Synthesize the following compound using...Ch. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - 37. Explain how and IR spectroscopy could be used...Ch. 12 - 38. An unknown X shows a broad absorption band in...Ch. 12 - Prob. 39PCh. 12 - The problem below is directed toward devising a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Whether an allele is dominant or recessive depends on
a. how common the allele is, relative to other alleles...
Campbell Biology: Concepts & Connections (9th Edition)
SSM WWW A lowly high diver pushes off horizontally with a speed of 2.00 m/s from the platform edge 10.0 m above...
Fundamentals of Physics Extended
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
a. Draw the mechanism for the following reaction if it a involves specific-base catalysis. b. Draw the mechanis...
Organic Chemistry (8th Edition)
Practice Exercise 2
By using a conversion factor from the back inside cover, determine the length in kilometer...
Chemistry: The Central Science (14th Edition)
Identify each of the rocks shown in Figure 2.26 and the rock group to which each belongs.
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br OH Brarrow_forwardQ7: Rank the following groups in order of basicity, nucleophilicity, and leaving group ability. a) H₂O, OH, CH3COOT b) NH3, H₂O, H₂Sarrow_forwardQ8: Rank the following compounds in order of increasing reactivity in a nucleophilic substitution reaction with CN as the nucleophile. Br A B NH2 LL F C D OH CI LLI E Q9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d). a) H "Cl D + -OCH 3 Page 3 of 5arrow_forward
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License