
A refrigerator operating on the vapor-compression refrigeration cycle using refrigerant-134a as the refrigerant is considered. The temperatures of the cooled space and the ambient air are at 10°F and 80°F, respectively. R-134a enters the compressor at 20 psia as a saturated vapor and leaves at 140 psia and 160°F. The refrigerant leaves the condenser as a saturated liquid. The rate of cooling provided by the system is 45,000 Btu/h. Determine (a) the mass flow rate of R-134a and the COP, (b) the exergy destruction in each component of the cycle and the second-law efficiency of the compressor, and (c) the second-law efficiency of the cycle and the total exergy destruction in the cycle.
(a)
The mass flow rate of R-134a and the COP.
Answer to Problem 30P
The mass flow rate of R-134a and the COP is
Explanation of Solution
Show the T-s diagram for vapor-compression refrigeration cycle as in Figure (1).
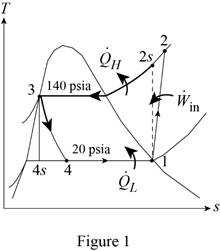
From Figure (1), write the specific enthalpy at state 3 is equal to state 4 due to throttling process.
Here, specific enthalpy at state 3 and 4 is
Express the work input.
Here, specific enthalpy at state 2 and 1 is
Express heat supplied to the cooled space.
Express the heat removed from the cooled space.
Express quality at state 4.
Here, specific enthalpy at saturated liquid and evaporation and
Express specific entropy at state 4.
Here, specific entropy at saturated liquid and evaporation and
Express mass flow rate of R-134a.
Here, rate of heat lost is
Express the COP of the cycle.
Conclusion:
Refer Table A-12E, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to initial pressure
Here, specific entropy at state 1 is
Refer Table A-13E, “superheated refrigerant-134a”, and write the properties corresponding to pressure at state 2
Here, specific entropy at state 2 is
Refer Table A-12E, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to pressure at state 3
Here, specific entropy at state 3 is
As specific enthalpy at state 3 is equal to specific enthalpy at state 4,
Refer Table A-12E, “saturated refrigerant-134a-pressure table”, and write the properties corresponding to pressure at state 4
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Hence, the mass flow rate of R-134a and the COP is
(b)
The exergy destruction in each component of the cycle and the second-law efficiency of the compressor.
Answer to Problem 30P
The exergy destruction in compressor is
Explanation of Solution
For compressor:
Express the exergy destruction in compressor.
Here, surrounding temperature is
For condenser:
Express the exergy destruction in condenser.
Here, entropy generation during process 2-3 is
For expansion valve:
For evaporator:
Express the exergy destruction in evaporator.
Here, entropy generation during process 4-1 is
Express the power input of the compressor.
Express second law efficiency of the compressor.
Conclusion:
Perform unit conversion of surrounding temperature from
Perform unit conversion of high temperature medium from
Perform unit conversion of low temperature medium from
Substitute
Hence, the exergy destruction in compressor is
Substitute
Hence, the exergy destruction in condenser is
Substitute
Hence, the exergy destruction in expansion valve is
Substitute
Hence, the exergy destruction in evaporator is
Substitute
Substitute
Hence, the second-law efficiency of the compressor is
(c)
The second-law efficiency of the cycle and the total exergy destruction in the cycle.
Answer to Problem 30P
The second-law efficiency of the cycle is
Explanation of Solution
Express the exergy of the heat transferred from the low temperature medium.
Determine the second law efficiency of the cycle.
Express the total exergy destruction in the cycle.
Conclusion:
Substitute
Substitute
Hence, the second-law efficiency of the cycle is
Substitute
Hence, the total exergy destruction in the cycle is
Want to see more full solutions like this?
Chapter 11 Solutions
Thermodynamics: An Engineering Approach
- During some actual expansion and compression processes in piston–cylinder devices, the gases have been observed to satisfy the relationship PVn = C, where n and C are constants. Calculate the work done when a gas expands from 350 kPa and 0.03 m3 to a final volume of 0.2 m3 for the case of n = 1.5. The work done in this case is kJ.arrow_forwardCarbon dioxide contained in a piston–cylinder device is compressed from 0.3 to 0.1 m3. During the process, the pressure and volume are related by P = aV–2, where a = 6 kPa·m6. Calculate the work done on carbon dioxide during this process. The work done on carbon dioxide during this process is kJ.arrow_forwardThe volume of 1 kg of helium in a piston–cylinder device is initially 5 m3. Now helium is compressed to 3 m3 while its pressure is maintained constant at 130 kPa. Determine the initial and final temperatures of helium as well as the work required to compress it, in kJ. The gas constant of helium is R = 2.0769 kJ/kg·K. The initial temperature of helium is K. The final temperature of helium is K. The work required to compress helium is kJ.arrow_forward
- A piston-cylinder device initially contains 0.4 kg of nitrogen gas at 160 kPa and 140°C. Nitrogen is now expanded isothermally to a pressure of 80 kPa. Determine the boundary work done during this process. The properties of nitrogen are R= 0.2968 kJ/kg-K and k= 1.4. N₂ 160 kPa 140°C The boundary work done during this process is KJ.arrow_forward! Required information An abrasive cutoff wheel has a diameter of 5 in, is 1/16 in thick, and has a 3/4-in bore. The wheel weighs 4.80 oz and runs at 11,700 rev/min. The wheel material is isotropic, with a Poisson's ratio of 0.20, and has an ultimate strength of 12 kpsi. Choose the correct equation from the following options: Multiple Choice о σmax= (314) (4r2 — r²) - о σmax = p² (3+) (4r² + r²) 16 σmax = (314) (4r² + r²) σmax = (314) (4² - r²)arrow_forwardI don't know how to solve thisarrow_forward
- I am not able to solve this question. Each part doesn't make sense to me.arrow_forwardExercises Find the solution of the following Differential Equations 1) y" + y = 3x² 3) "+2y+3y=27x 5) y"+y=6sin(x) 7) y"+4y+4y = 18 cosh(x) 9) (4)-5y"+4y = 10 cos(x) 11) y"+y=x²+x 13) y"-2y+y=e* 15) y+2y"-y'-2y=1-4x³ 2) y"+2y' + y = x² 4) "+y=-30 sin(4x) 6) y"+4y+3y=sin(x)+2 cos(x) 8) y"-2y+2y= 2e* cos(x) 10) y+y-2y=3e* 12) y"-y=e* 14) y"+y+y=x+4x³ +12x² 16) y"-2y+2y=2e* cos(x)arrow_forwardQu. 15 What are the indices for the Plane 1 drawn in the following sketch? Qu. 16 What are the Miller indices for the Plane shown in the following cubic unit cell? this is material engineering please show all workarrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
