
A bomb calorimeter is a study metal vessel in which samples can be ignited and the amount of heat given off can be measured as the heat warms up surrounding water. Draw a rough sketch of such an experimental setup and label (a) the system and (b) the surroundings.
Interpretation:
A rough sketch of experimental setup of bomb calorimeter is to be drawn with labeling of (a) system and (b) surroundings
Concept introduction:
Calorimeter is an instrument which is used to measure the heat of the chemical reactions, physical changes and heat capacity. There are different types of calorimeter such as 1) differential scanning calorimeter 2) isothermal calorimeter 3) titration calorimeter 4) accelerated rate calorimeter. The main components of calorimeter consist of a thermometer attached to a metal container with water suspended above a combustion chamber. This device is used in various fields to study thermodynamics, chemistry and biochemistry.
Answer to Problem 1.1E
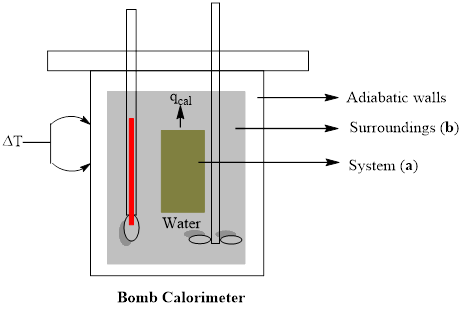
Explanation of Solution
A bomb calorimeter is a kind of constant-volume calorimeter, which is used to measure the heat of combustion of a particular reaction. The four important components of bomb calorimeter are as follows; 1) a bomb (vessel) having the combustible material. It must be a strong and thick-walled vessel which is made up of high-quality metal and should have a provision to open for inserting the sample, to remove the products of combustion and to clean the apparatus. 2) a bucket (container) with a measured quantity of water to hold the bomb. Besides, it should be fitted with a probe to read the temperature and a mechanical stirrer to promote rapid thermal equilibrium with no excessive heat in the form of mechanical energy. These buckets are made with a highly polished exterior finish to reduce the absorption and emission of heat as radiation. 3) An insulating jacket to protect the container from transient thermal changes during the combustion process
The enthalpy change per mole of a substance A in a reaction between A and B is measured by adding the substances separately into the calorimeter and calculating the initial and final temperatures of the system. On multiplying the temperature change by the mass and specific heat capacities of the substances, we can get the value of energy which is given off during the reaction. When we divide the energy change by moles of A involved in the reaction, we can obtain the enthalpy change of reaction.
A rough sketch of experimental setup of bomb calorimeter is drawn with labeling of (a) system and (b) surroundings.
Want to see more full solutions like this?
Chapter 1 Solutions
Physical Chemistry
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





