
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1, Problem 1.30E
Determine the expressions for the following, assuming that the
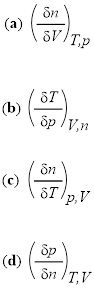
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
True or false, chemistry
answer thse questions with mechanisms and steps. handwritten please!
C
app.aktiv.com
Draw the product of the following reaction sequence. Ignore
any inorganic byproducts formed.
H
O
1. (CH3CH2)2CuLi, THF
2. CH3Br
Drawing
Chapter 1 Solutions
Physical Chemistry
Ch. 1 - A bomb calorimeter is a study metal vessel in...Ch. 1 - Difference between the system and the...Ch. 1 - Prob. 1.3ECh. 1 - Prob. 1.4ECh. 1 - Prob. 1.5ECh. 1 - Prob. 1.6ECh. 1 - Prob. 1.7ECh. 1 - A pot of cold water is heated on a stove, and when...Ch. 1 - hat difference is necessary for heat to flow...Ch. 1 - What is the value of FT for a sample of gas whose...
Ch. 1 - What is the value of FP for a sample of gas whose...Ch. 1 - Prob. 1.12ECh. 1 - Hydrogen gas is used in weather balloon because it...Ch. 1 - Prob. 1.14ECh. 1 - A 2.0 L soda bottle is pressurized with 4.5 atm of...Ch. 1 - The Mount Pinatubo volcano eruption in 1991...Ch. 1 - Prob. 1.17ECh. 1 - Scottish physicist W. J. M. Rankine proposed an...Ch. 1 - Use the two appropriate values of R to determine a...Ch. 1 - Prob. 1.20ECh. 1 - Pressures of gases in mixtures are referred to as...Ch. 1 - Earths atmosphere is approximately 80 N2 and 20...Ch. 1 - The atmospheric surface pressure on Venus is 90...Ch. 1 - Prob. 1.24ECh. 1 - Prob. 1.25ECh. 1 - In the anaerobic oxidation of glucose by yeast,...Ch. 1 - What are the slopes of the following lines at the...Ch. 1 - For the following function, evaluate the...Ch. 1 - Determine the expressions for the following,...Ch. 1 - Determine the expressions for the following,...Ch. 1 - Prob. 1.31ECh. 1 - Prob. 1.32ECh. 1 - Prob. 1.33ECh. 1 - Prob. 1.34ECh. 1 - What properties of a nonideal gas do the Vander...Ch. 1 - Prob. 1.36ECh. 1 - Prob. 1.37ECh. 1 - Calculate the Boyle temperatures for carbon...Ch. 1 - Prob. 1.39ECh. 1 - Prob. 1.40ECh. 1 - Table 1.4 show that the second virial coefficient...Ch. 1 - Prob. 1.42ECh. 1 - What is the van der Waals constant a for Ne in...Ch. 1 - Prob. 1.44ECh. 1 - Under what conditions would the van der Waals...Ch. 1 - By definition, the compressibility of an ideal gas...Ch. 1 - The second virial coefficient B and the third...Ch. 1 - Use the approximation 1 x-1 1 x x2 to...Ch. 1 - Why is nitrogen a good choice for the study of...Ch. 1 - Evaluate for a gas following the Redlich-Kwong...Ch. 1 - Numerically evaluate for one mole of methane...Ch. 1 - Under what conditions of volume does a van der...Ch. 1 - At high temperatures, one of the van der Waals...Ch. 1 - Under what conditions of temperature does a...Ch. 1 - The Berthelot equation of state for one mole of...Ch. 1 - Prob. 1.56ECh. 1 - Referring to exercises 1.6 and 1.7, does it matter...Ch. 1 - Prob. 1.58ECh. 1 - Use Figure 1.11 to construct the cyclic rule...Ch. 1 - Prob. 1.60ECh. 1 - Prob. 1.61ECh. 1 - Calculate for one mole of an ideal gas at STP and...Ch. 1 - Prob. 1.63ECh. 1 - Show that = T/p for an ideal gas.Ch. 1 - Determine an expression for V/T p, n in terms of ...Ch. 1 - Prob. 1.66ECh. 1 - Prob. 1.67ECh. 1 - Perform a units analysis on the exponent of the...Ch. 1 - Using the barometric formula, calculate the...Ch. 1 - The barometric formula can also be used for...Ch. 1 - Prob. 1.71ECh. 1 - Prob. 1.72ECh. 1 - Prob. 1.73ECh. 1 - Prob. 1.74ECh. 1 - Prob. 1.75ECh. 1 - Prob. 1.76ECh. 1 - Prob. 1.77ECh. 1 - Prob. 1.78ECh. 1 - Prob. 1.79ECh. 1 - Use the ideal gas law to symbolically prove the...Ch. 1 - Prob. 1.81E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the mechanismarrow_forwardV Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
DISTINCTION BETWEEN ADSORPTION AND ABSORPTION; Author: 7activestudio;https://www.youtube.com/watch?v=vbWRuSk-BhE;License: Standard YouTube License, CC-BY
Difference Between Absorption and Adsorption - Surface Chemistry - Chemistry Class 11; Author: Ekeeda;https://www.youtube.com/watch?v=e7Ql2ZElgc0;License: Standard Youtube License